Fig. 1.
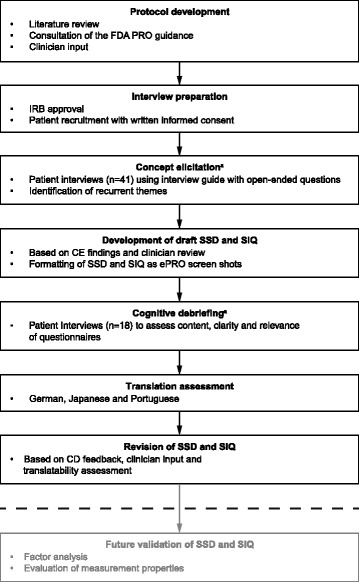
PRO development process. aDistinct groups of patients participated in the CE and CD interviews. CD, cognitive debriefing; CE, concept elicitation; ePRO, electronic patient reported outcome; FDA, Food and Drug Administration; IRB, internal review board; PRO, patient-reported outcome; SIQ, SLE Impact Questionnaire; SSD, SLE Symptom Severity Diary
