Abstract
To determine whether human whole semen (WS) and seminal plasma (SP) either previously frozen or freshly acquired altered ex vivo infectibility of human colonic explants or was associated with histology or toxicity changes, which may influence mucosal HIV-1 transmission in vivo. Pooled human semen samples were freshly obtained from study volunteers (never frozen) and from commercial sources (frozen/thawed). Endoscopically acquired rectal biopsies were evaluated for toxicity following titered ex vivo WS/SP exposure by histological grading and by MTT assay. The ex vivo HIV-1 biopsy challenge model was used to evaluate effects of exposure to either previously frozen or freshly acquired WS/SP on HIVBaL infectibility at a range of viral inocula (104–100 TCID50). To evaluate the effects at lower viral inocula of HIV-1 (10−2–102), experiments in the presence or absence of WS/SP were also performed utilizing TZM-bl cells. MTT assays and histological scoring demonstrated no tissue degradation of biopsies when exposed for 2 h to concentrations of 10% or 100% of either fresh or previously frozen WS/SP. Ex vivo biopsy HIV-1 challenge experiments showed no differences in the presence of freshly acquired or previously frozen/thawed WS/SP compared with control; no differences were seen with lower infectious titers on TZM-bl cells. Within the limits of assay sensitivity and variability, these data show no toxicity or significant enhancement of HIV-1 infectibility of human rectal mucosa using the colorectal explant model with either pooled fresh or frozen/thawed nonautologous human semen.
Keywords: : semen, seminal plasma, rectal, mucosa, HIV, explants
Introduction
Semen is the most common vector for delivery of HIV-1 into the anogenital compartments.1,2 Semen contains cell-free virions and seminal-infected leukocytes1,3 with continued debate over which component is more infectious. While emerging data provide compartment-specific and behavioral factors that significantly impact HIV-1 transmission and infection,4,5 the role of semen or seminal products as an active contributor to enhancing infection, not just a mode of delivery, has been under debate. Conflicting in vitro and in vivo studies have demonstrated inhibitory,6–10 enhancing,11–16 and no effect17,18 of semen or seminal plasma (SP) on HIV-1 transmission. These discordant findings might be derived from varying experimental approaches, but reports have not addressed directly the clinically relevant effects of freshly acquired ejaculate in altering ex vivo infectibility profiles using freshly acquired human colorectal biopsies.
Despite sexual transmission being the most common mode of viral transmission, there is a low relative incidence of transmission of HIV-1 per sexual act (138:10,000 per receptive anal intercourse and 8:10,000 per vaginal sex act).4,5,19,20 SP has been demonstrated to inhibit HIV transmission by preventing HIV-1 recognition, attachment, and transmission to CD4+ T cells by DCs and DC-SIGN-expressing cells in various in vitro cell lines.6,8 SP contains cationic polypeptides with intrinsic antiviral activity7 and leukocyte toxicity that inhibits HIV-1 infection.9
Amyloidogenic peptides identified as semen-derived enhancers of virus infection (SEVI) were shown to significantly enhance HIV-1 infectivity in vitro with exposure to low viral doses.11 Prostatic acid phosphatase (PAP), such as PAP248–286 or PAP85–120, and semenogelin-derived fragments in semen form amyloid fibrils that were discovered to enhance HIV infection up to 105-fold in various cell culture models.12,13,15,21,22 These amyloid fibrils are thought to promote the binding of HIV-1 to target cells.21,22 In addition, microbicide antiviral efficacy, with the exception of maraviroc, was decreased in TZM-bl cell lines when exposed to semen.16 In most of these studies, semen/seminal products were acquired frozen from commercial sources and then thawed for experimental studies.11–13,15,21,22 Other experiments saw no effect on antiviral potency in animal or human reproductive tissue explants.17,18 Negative findings were noted when applying semen to colonic tissue explants.23,24 Importantly, while a very different mucosal compartment, enhancement of HIV-1 replication was noted in a recent study, which utilized an ex vivo ectocervical explant model and SP.25
More direct, clinically relevant studies to evaluate whether semen and semen components enhance HIV-infectibility of the more vulnerable colorectal mucosa using freshly acquired human ejaculate and human colorectal biopsies are imperative given the variable influences of semen on HIV-1 transmission. Within the limits of the assays currently available, we hypothesized that titered exposures of freshly acquired whole semen (WS) and SP would have no detectable influence on tissue integrity or ex vivo infectibility of human colonic explants using wide ranges of TCID50 HIVBaL (including TZM-bl cell lines for lower titers). Commercially available frozen human semen (thawed for experiments) was included to enable cross-study comparisons.
Methods
Subjects and sources of semen and colorectal tissue biopsies
HIV-seronegative subjects were recruited from the Mucosal Immunology Core Laboratory (MICL) Registry for biopsies and fresh semen collection. All participants gave informed consent and the IRB at the David Geffen School of Medicine at UCLA, Los Angeles, California approved the research protocol.
Fresh semen collection
HIV-seronegative men recruited from UCLA were provided with a specimen collection kit that included a sterile specimen collection cup (VWR San Francisco, CA) and a ziplock bag for returning sample on ice. Donors were asked to refrain from ejaculation for a period of 48 h before providing samples and to deliver the sample to the laboratory within 2 h of ejaculation. The fresh samples ranged from 0.5 to 7 ml and were used in experiments within 2 h of delivery. Samples collected from three donors on the same day were pooled and divided in half. Half of the unfractionated WS was maintained on ice, whereas the remainder was placed in a microcentrifuge at 10,000 rpm for 10 min to obtain the clarified supernatant or SP. This was separated to an additional Eppendorf tube and either maintained on ice before experiments or stored at −80 for future experiments.
Frozen WS
Frozen WS was acquired from Lee Biosolutions, Inc. (www.leebio.com). At collection per their protocol, single and pooled samples were frozen (−20°C) within 5 min after acquisition without dilution (averaged 2–8 ml) or added cryoprotectants. Before all experiments, the frozen WS samples were thawed to room temperature.
Fresh and frozen/thawed semen dilutions were prepared in explant medium (RPMI 1640; Invitrogen, Carlsbad, CA) supplemented with 0.50 mg/ml Zosyn (Wyeth, Philadelphia, PA) and 1.25 μg/ml amphotericin-B (Invitrogen).
Biopsy acquisition for explant studies
Rectosigmoid biopsies were collected endoscopically using a flexible sigmoidoscope as previously described.26 The rectosigmoid colon was sampled 10–30 cm from the anal verge, and tissue (∼30 biopsies per subject) was placed immediately in 25 ml of RPMI supplemented with 10% FCS (Invitrogen), 1.25 μg/ml amphotericin-B (Invitrogen), and 0.50 mg/ml Zosyn (Wyeth) per routine. Tissue was maintained at room temperature and transported to the laboratory within 1 h of collection for immediate use for histology, MTT assay, and explant studies.
Virology
HIVBaL virus and PM1 cells were acquired from the NIH AIDS Repository (Bethesda, Maryland). The viral stock was expanded using PM1 cells. Viral TCID50 was determined using human PBMC as previously reported.27 The same HIV-1BaL viral stocks in varying TCID50 titers were used in all biopsy infections (100, 101, 102, 103, and 104) and TZM-bl (10−2–102) experiments.
Histopathology
Biopsies from six individuals were exposed to previously frozen WS/SP, and four individuals provided biopsies for freshly acquired WS and SP exposure at a final concentration of 10%, 50%, and 100% for 2 h at 37°C and 5% CO2 to evaluate histology changes in semen-exposed samples. For histology, biopsies were mounted on mesh screens, fixed in 10% formalin, and embedded in paraffin, from which 7 micron sections were hematoxylin and eosin stained for qualitative histological assessments by a pathologist with specialty training in gastrointestinal pathology, who was blinded to experimental conditions. A qualitative score of chronic active inflammation, ranging from 1 (normal) to 6 (mucosal erosion or ulceration) was assigned.28
MTT assay
Biopsies in duplicate from 10 individuals were exposed to media alone (controls), frozen/thawed WS (10%, 100%), frozen/thawed SP (10%, 50%, 100%), and fresh WS or SP (50%). WS and SP were diluted in explant medium for 2 h at 37°C and 5% CO2, mirroring the first 24 h of explant tissue exposure. For determination of viability using the MTT assay (Chemicon, Temecula, CA), biopsy explants were gently blotted, weighed, and transferred to a 96-well plate containing 100 μl of MTT (500 μl/ml) for 3 h at 37°C before being transferred to 500 μl of methanol in 1.5 ml Eppendorf tubes (Denville Scientific, Metuchen, NJ) and incubated overnight at room temperature in the dark. The absorbance of the MTT-formazan product was measured at 570 nm (corrected at 630 nm). The viability was determined by dividing absorbance by the weight of each biopsy. The effect of semen and SP on the tissue viability was calculated as a percentage compared to the experimental media controls. Tissue viability was defined as >40% of controls.
Ex vivo biopsy HIV-1 challenge
Twelve individuals provided endoscopic biopsies. Biopsies in fresh WS/SP (50%) were submerged in the presence of HIV-1BaL TCID50 of 101, 102, and 103 in a total volume of 500 μl. Tissues in frozen-then-thawed WS/SP (100%, 10%) were challenged in the presence of HIV-1BaL TCID50 of 102 and 104 in a final volume of 500 μl. Following a 2 h incubation at 37°C under 5% CO2, biopsies were washed five times in RPMI-1640 supplemented with 10% FCS (Invitrogen) 1.25 μg/ml amphotericin-B (Invitrogen) and 0.50 mg/ml Zosyn (Wyeth) and established in culture on top of surgifoam rafts (Ferrosan, Soeborg, Denmark) as previously described.28 Supernatants were collected and stored at −30°C on days 1, 4, 7, 11, and 14 postexposure. Viral replication was determined by quantitation of p24 (pg/ml) in the culture supernatants at each time point as previously reported,28 using the HIV-1 Antigen Capture Assay Kit (AIDS Vaccine Program, NCI-Frederick Cancer Research and Development Center, Frederick, MD).
TZM-bl studies
Cells (NIH AIDS repository) were propagated in DMEM (Invitrogen) supplemented with 10% FBS and 1% Streptomycin/Penicillin (Invitrogen) in a 25 ml vented flask in a 37°C 5% CO2 incubator. Following trypsinization (Invitrogen), cells were plated at 4,000 cells/well in a 96-well plate and allowed to adhere overnight in a growth medium in the incubator. The following day, the medium was removed and the cells were incubated in the presence HIV-1BaL titers ranging from 10−2 to 102 TCID50 in combination 1%–10% concentrations of either frozen or freshly acquired WS or SP. After 2 h, the cells were washed twice with PBS and placed in a growth medium for 48 h at 37°C in a humidified 5% CO2 incubator. Cells were lysed with Promega Cell Culture Lysis buffer part E153A. HIV replication was measured utilizing the Promega Luciferase Assay Kit (Part No. 1501) following the manufacturer's instructions. Relative luminescence units (RLU) were determined using Optima software by BMG Labtech read on a FLUOstar plate reader.
Statistical analyses
Permutation test was utilized for discrete histology data. Two-sided paired t-test was used for the MTT data; p-values <.1 were considered to be statistically significant.
For viral replication differences in ex vivo explant infection studies, p24 measurements below the limit of quantification (100 pg/ml) were imputed as 100 pg/ml and were log transformed. The percent coefficient of variation (% CV) was used to measure variability across replicate measurements. Equivalence between WS or SP and control conditions was tested per assay type (tissue explant and TZM-bl), WS/SP treatment (fresh or frozen, 10%–100% concentrations), HIV-1BaL infection (10–104 TCID50) and, for the tissue explant assay results, per day following ex vivo infection. The WS or SP and control conditions were considered to be equivalent when a 1:1 ratio (treated:control) was included in the confidence interval (95% CI).
Ex vivo p24 data were modeled using a nonlinear three-parameter curve for each condition (WS, SP, and control) and HIV-1BaL titer (10–104 TCID50).
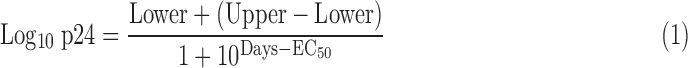 |
The three curve parameters (lower, upper, and EC50) for the WS and SP conditions were compared to control results by F test to determine whether these predefined groups differ from each other.
Results
Subject characteristics
For the freshly acquired semen samples, 25 healthy HIV-seronegative men contributed samples [mean age: 40.2 years; Caucasian (32%), Asian (32%), African American (24%), and Hispanic or Latino (4%)] (Table 1). For the freshly acquired colorectal tissue biopsies, 29 healthy HIV-seronegative participants underwent sigmoidoscopic biopsies to donate tissue [mean age: 43.9 years; 61% male, 39% female; Caucasian (24%), African American (59%), and Hispanic or Latino (14%)]. There were no serious or procedure-related adverse events. Due to the limited number of biopsies available per subject (maximum of 30 biopsies per subject), sufficient tissues to perform all assays with all combinations of WS or SP at multiple viral titers was not possible. Each subject was his own control.
Table 1.
Baseline Characteristics of Each Enrolled Group
| Tissue | Fresh semena | |
|---|---|---|
| Participants enrolled | 29 | 25 |
| Mean age in years (SD) | 43.9 (10) | 40.2 (11.6) |
| Gender, n (%) | ||
| Male | 20 (69) | 25 (100) |
| Female | 9 (31) | |
| Race, n (%) | ||
| Hispanic origin or Latino | 4 (14) | 1 (4) |
| Caucasian | 7 (24) | 8 (33) |
| Asian | 0 (0) | 8 (33) |
| African American | 17 (59) | 6 (24) |
| Other or unknown | 1 (3) | 2 (8) |
HIV-seronegative participants contributed freshly acquired semen (n = 25 men, mean age 43.9 years) and colonic tissue biopsies during sigmoidoscopy (n = 29 men and women, mean 40.2 years). The men who contributed biopsies were different than men who donated semen.
Purchased pooled frozen semen not included.
Neither fresh nor frozen-then-thawed WS or SP induced histological change in fresh colonic biopsies ex vivo
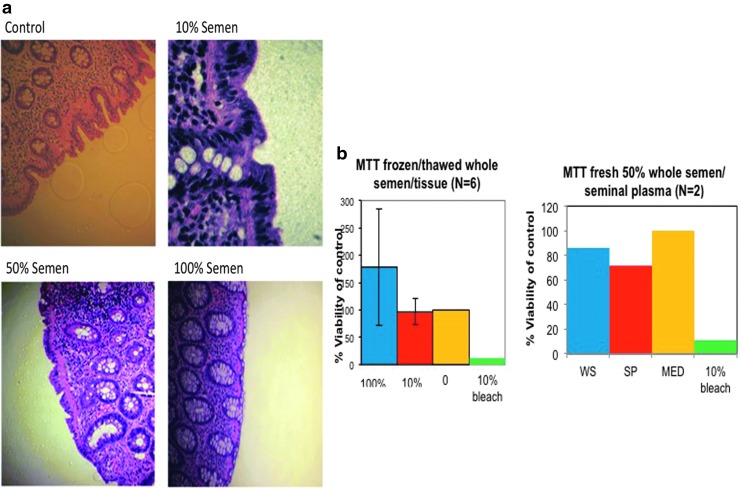
Biopsies from healthy nonsemen donor subjects were incubated for 2 h in either previously frozen-then-thawed (N = 6 subjects) or freshly acquired WS (N = 4 subjects) or SP (N = 4 subjects) A single-blinded pathologist performed the qualitative histological assessments to characterize potential tissue-associated injury when exposed to various conditions of WS (10%, 25%, 50%, 100%) or SP (10%, 100%). Scoring ranged from 1 (normal) to 6 (mucosal erosion or ulceration). Overall, no tissue deterioration was observed with either frozen-then-thawed or fresh WS or SP compared to media (Fig. 1a).
FIG. 1.
No alteration of histology and viability of colonic tissue exposed to fresh or frozen-then-thawed WS and SP. (a) Immersion of freshly acquired colonic tissue for 2 h in various concentrations of fresh or frozen-then-thawed WS (10%, 25%, 50%, 100%) and SP (10%, 100%) did not significantly induce colonic tissue histology changes ex vivo as assessed by a single-blinded pathologist. All of the tissues in 100% semen concentration scored 1–2, mean event rate 2 (0). The tissues in 10% WS or SP scored 1–3, mean event rate of 1.78 (0.67). Tissue in control condition without semen or SP scored 1–3, mean event rate 1.9 (0.73). (b) The effect of semen and SP on tissue viability in the MTT assay was defined as >40% compared to the experimental media controls. No reduction of tissue viability below 40% was noted after 2 h exposure to frozen-then-thawed WS and SP (10%, 50%, 100%) nor fresh WS or SP (50%). SP, seminal plasma; WS, whole semen.
Colorectal mucosal samples exposed to WS or SP do not show increased toxicity by MTT readouts
Using the MTT assay as a readout for tissue toxicity, fresh biopsies in duplicate were exposed for 2 h, to approximate some clinical relationship, to 10%, 50%, 100% frozen/thawed WS or SP, or to 50% fresh WS or SP. A solution of 10% bleach was included as a positive control. Exposed biopsies were then washed and cultured overnight. The MTT (OD units per mg of tissue) mean (SD) of 100% frozen–thawed WS is 56.9 (43.3), 10% frozen–thawed WS is 33.5 (32.3), median 38.2 (24.5). Data are reported as a percentage of tissue exposed to media. Using a two-sided paired t-test, no significant differences between media control and 10% frozen–thawed WS or between media control and 100% frozen–thawed WS were seen. There was insufficient power to test a difference using fresh semen/tissue samples. However, no tissue sample in any group was below the 40% threshold, which is considered to represent decreased viability (Fig. 1b).
No significant differences of ex vivo HIV-1 explant tissue infections were seen between fresh and frozen-then-thawed WS or SP
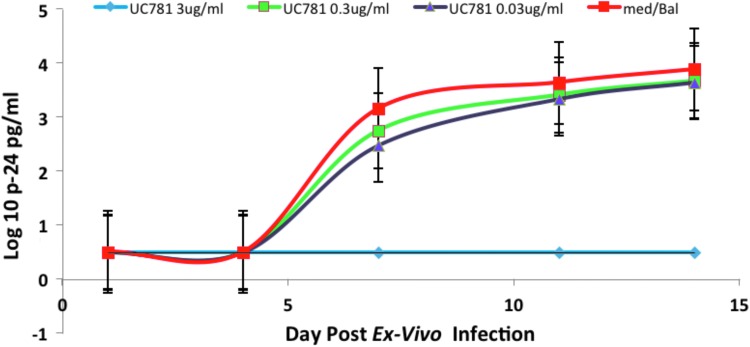
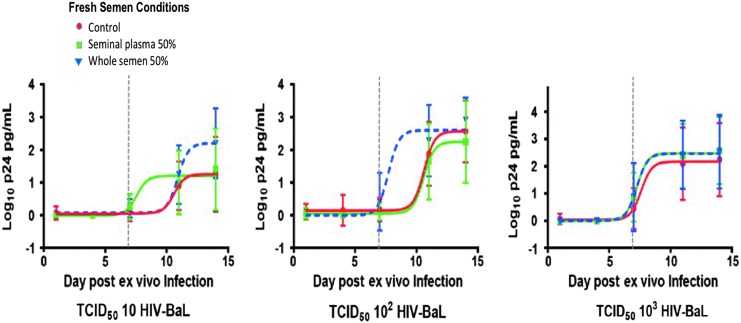
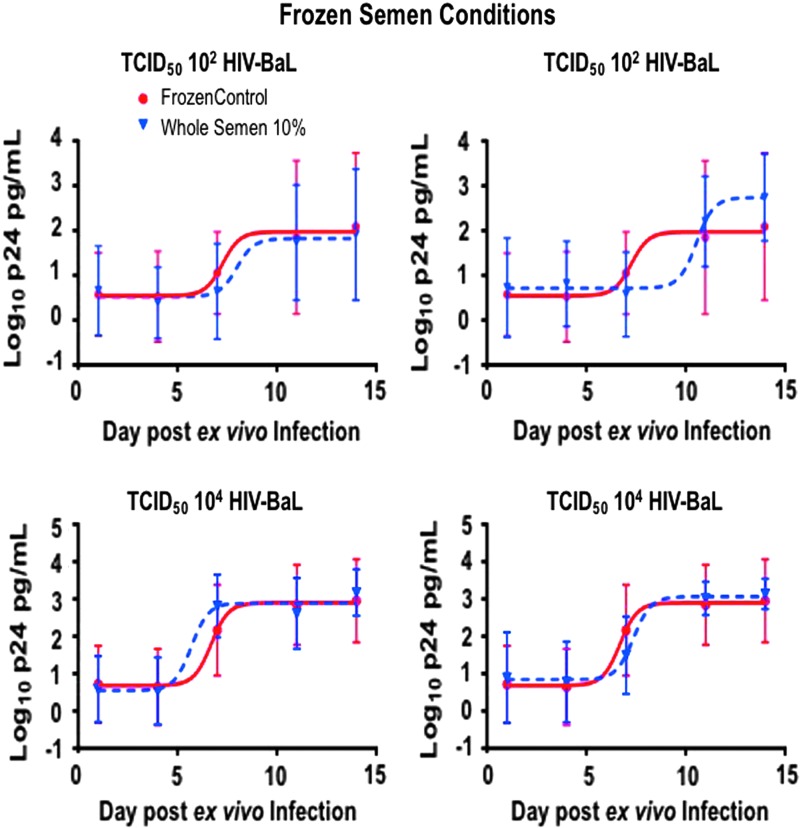
Biopsies in triplicate were incubated ex vivo for 2 h with either 10% or 100% of pooled, freshly acquired WS and SP (N = 12; n = 36), frozen-then-thawed WS (N = 5; n = 15), or frozen-then-thawed SP (N = 6; n = 18). All tissues were incubated with a pool of three subjects' ejaculate. To reduce intrasubject variation, experiments for fresh and frozen WS/SP conditions were conducted with biopsies acquired from the same subjects. Infection was determined by cumulative p24 in culture supernatants after 14 days of culture, which has become the standard method for reporting ex vivo explant challenge data.24,25,27,29–31 Utilization of this colorectal explant assay in previous experiments has demonstrated adequate p24 readouts with complete suppression of viral replication in the setting of a potent high-dose nonnucleoside reverse transcriptase inhibitor, UC-781, compared to explant in media alone (Fig. 2). In the semen experiments, colorectal explants exposed to fresh 50% WS and SP regardless of viral titers (10, 102, and 103 TCID50 of HIVBaL) had similar p24 profiles to colorectal tissue without semen by the end of the 2-week assay (odds ratio 1.06–0.93, 95% CI) (Fig. 3). Colorectal explants in triplicate exposed to frozen-then-thawed WS (N = 5; n = 15) and SP (N = 6; n = 18) challenged with 102 and 104 TCID50 of HIVBaL did not show significant differences in p24 profiles by day 14 (odds ratio 0.75–0.99, 95% CI) (Fig. 4).
FIG. 2.
Colorectal explants (n = 3) were incubated with UC781 (0.03–3 μg/ml) or medium alone for 2 h. Following a 2-h infection with HIV-1 BaL (TCID50 103) ex vivo explant cultures were established as described and culture medium exchanged every 3–4 days for 14 days. P24 was assayed in the culture supernatant. The plot represents the combined cumulative p24 data obtained from N = 3 subjects with standard error bars.
FIG. 3.
Cumulative p24 (log10) viral concentrations in HIV-exposed rectal explants with either fresh 50% WS or SP were similar by the end of the 2-week assay (odds ratio 1.06–0.93, 95% CI). In conditions of fresh 50% WS, the EC50 was reached at 7.7 days following 102 TCID50 infection compared with the SP and control (10–11 days) (p = .02). At day 14, there were no significant differences in p24 with low to high TCID50 exposure. Mean ± SD plotted with nonlinear curve.
FIG. 4.
No significant differences in p24 profiles by day 14 in colorectal tissue exposed to frozen-then-thawed WS and SP challenged with 102 and 104 TCID50 of HIVBaL (odds ratio 0.75–0.99, 95% CI). Mean ± SD plotted with nonlinear curve.
Fresh WS and not frozen semen or seminal derivatives suggests earlier ex vivo explant kinetics
A trend was observed during the early assay phase of ex vivo explant infection in that explants exposed to fresh 50% WS and SP trended to have an earlier rise in p24 levels (odds ratio 1.08–2.45, 95% CI). Following infection with 102 TCID50 HIVBaL, the EC50 was reached at 7.7 days compared with the SP and control (10–11 days) (p = .02). No significant differences were appreciated in the kinetics of p24 production, when explants were exposed to frozen semen or seminal derivatives.
No enhancing effect on HIV-1 infectibility was seen in TZM-bl cell lines using fresh or frozen-then-thawed WS or SP
Viral titers less than 101 TCID50 are not useful in ex vivo explant assays to evaluate infectibility.28 Previous studies demonstrated that in the setting of seminal products and low viral concentrations, SEVI enhanced HIV-1 infection in various cell lines, including TZM-bl.11–15 Consequently, we used fresh TZM-bl cell lines exposed to progressively lower TCID50 of the same HIV-1 viral stock, using varying conditions of both fresh and frozen/thawed WS and SP as above. Cell line experiments focused on the early phase of infectibility, assessing p24 at 48 h, using low viral titers (0, 0.01, 0.1, 1, 101, and 102) added to TZM-bl cells incubated with 1% and 10% concentrations of WS or SP. No enhancing effect of WS or SP on HIV infectibility was observed using either fresh or frozen/thawed WS or SP at any concentration. There were no significant differences on RLU in either WS or SP assays with the various viral titers (odds ratio fresh WS/SP 0.86–1.18, frozen-then-thawed WS/SP 0.86–1.06, 95% CI).
Discussion
In this report using freshly acquired human colorectal tissue biopsies and freshly acquired semen/SP for the first time, we attempted to determine whether freshly acquired or frozen-then-thawed seminal products might increase HIV-1 infectibility of colorectal mucosa. We incorporated well-established assays for ex vivo infection of freshly acquired rectal biopsies exposed to nonautologous freshly acquired semen or SP as well as products from the freeze–thaw process required with purchased semen. The results in clinically relevant samples showed no significant toxicity nor enhanced tissue infectivity by HIV-1BaL (100–103), regardless of whether fresh or frozen/thawed WS or SP was used. Similarly, using the same conditions with TZM-bl cells, which enable assessing lower viral titers (10−2–102), given the inability to detect reproducible tissue explant infections using lower viral titers,17,32 no semen/SP effects were seen using either fresh or frozen–thawed samples.
The role of semen in HIV-1 transmission remains conflicted, with intriguing evidence of enhancing11–16 effects. In cell culture using established protocols,11 artificially formed SEVI increases infectivity when exposed to low concentration of virus. Amyloid fibrils of human semen bind HIV-1 virions and neutralize the negative charge repulsion between virions and target cells. This is thought to increase the ability of HIV-1 to interact with the target cell surface in vitro,14 enhancing cellular infection. Semen is also reported to induce chemokines and cytokines, leading to robust recruitment of macrophages, dendritic cells, and memory T cells in the reproductive tract epithelia.24,29 This semen-induced proinflammatory condition may alter the activation state of target cells in human tissue at lower viral titers.3
Differences in our findings compared to reports on SEVI-enhancing HIV-1 replication may be attributed to variation in techniques and the source of tissue specimens. In the original experiments describing SEVI,11 purified seminal amyloid fibrils in frozen-then-thawed conditions were utilized on a variety of cell lines and ex vivo tonsillar tissues. In conditions of subinfectious X4-tropic HIV-1 (0.1 pg p24) and higher concentrations of SEVI (5–20 μg/ml) in tonsillar tissue, viral replication was induced with p24 rising after day 12 postinfection. Of interest, while most reports utilized frozen–thawed semen, another study in TZM-bl cell cultures exposed to fresh ejaculate found enhancement of infection.12 Both showed enhancement of infection, while our experiments did not.
In contrast, we utilized a large number of freshly acquired human biopsies in multiple experiments to attempt to simulate rectal compartment exposure to a range of HIV-1 dilutions known to infect colorectal explants, using varying concentrations of either fresh or frozen/thawed WS and SP. The range of concentrations used for seminal products was based somewhat on the unpredictable dilution effects in the rectal compartment in vivo).33 Following 14 days of culture, no differences were seen regardless of the semen source or derivative over a wide range of viral concentrations.
These results using freshly acquired human colorectal tissue and fresh as well as frozen/thawed semen showing lack of seminal enhancement of HIV-1 are similar to others utilizing cell lines, explants, and animal models.6–10,17,34,35 In human colonic explants, these findings are similar to those by Dezzutti et al.,34 which showed no enhanced infection. Interestingly, rectosigmoid tissue is more prone than tonsillar and cervical tissue to R5 HIV-1 infection due to the high prevalence of available R5 cell targets and reduced chemokine blockade,36,37 However, despite this increased gut lymphoid tissue infectibility, we did not see enhancement of viral replication early after HIV-1 exposure in fresh WS/SP (or frozen/thawed) conditions. This is likely a reflection of the limits with this ex vivo assay, which is too insensitive to confidently detect new infections in colorectal biopsies using HIV-1 infective titers less than 102 TCID50. However, knowing this, we utilized the same general study design, but replaced fresh biopsies with TZM-bl cell line targets for lower viral titer exposures with/without seminal products. Again, we saw no enhancement.
There are limitations to the explant HIV-1 infectibility assay related to sensitivity in detecting p24 in the early phase and by tissue decay in the late when maximally provoked, the ex vivo colorectal tissue assay may have detectable HIV-1 using both quantitative real-time-PCR and ELISA p24 readouts as early as day 4,36,38 however, the significance and reproducibility of these early phase p24 readouts using the ex vivo colorectal infection model have not been validated, requiring a caveat on these findings as well. Further development of more sensitive whole tissue assays utilizing well-characterized labeled viral vectors focusing on the first 24–48 h for viral integration and replication are underway and will help clarify the role of seminal products on enhancing clinically relevant mucosal target cells' infectibility.
Conclusion
In summary, the addition of freshly acquired or frozen/thawed semen/SP demonstrated no toxicity and did not alter the ex vivo HIV-1BaL infectibility of fresh colorectal biopsies using current explant and cell line assays. Similarly, no differences were seen using even lower infecting titers (10−2–102) of the same HIV-1BaL stock with the same seminal derivatives on the TZM-bl cell line. These findings need to be confirmed when there are more sensitive assays for early tissue infectibility post-HIV exposure.
Acknowledgments
This study was funded by an Integrated Preclinical/Clinical Program (IP/CP) U19 grant (AI060614) from the Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health with support from the UCLA AIDS Institute's Center for AID Research (CFAR) Mucosal Immunology and Virology Cores (AI28697). We thank Nicola Richardson-Harman of Alpha StatConsult, LLC in Damascus, MD for her critical contribution performing statistical analyses. Additional gratitude is extended to participants contributing tissue and semen samples for these studies through the UCLA CFAR MICL Participant Registry (part of the UCLA CFAR Mucosal Immunology Core).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Houzet L, Matusali G, Dejucq-Rainsford N: Origins of HIV-infected leukocytes and virions in semen. J Infect Dis 2014;210 Suppl 3:S622–S630 [DOI] [PubMed] [Google Scholar]
- 2.Royce RA, Sena A, Cates W, Jr., Cohen MS: Sexual transmission of HIV. N Engl J Med 1997;336:1072–1078 [DOI] [PubMed] [Google Scholar]
- 3.Doncel GF, Joseph T, Thurman AR: Role of semen in HIV-1 transmission: Inhibitor or facilitator? Am J Reprod Immunol 2011;65:292–301 [DOI] [PubMed] [Google Scholar]
- 4.Boily MC, Baggaley RF, Wang L, et al. : Heterosexual risk of HIV-1 infection per sexual act: Systematic review and meta-analysis of observational studies. Lancet Infect Dis 2009;9:118–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J: Estimating per-act HIV transmission risk: A systematic review. AIDS 2014;28:1509–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabatte J, Ceballos A, Raiden S, et al. : Human seminal plasma abrogates the capture and transmission of human immunodeficiency virus type 1 to CD4+ T cells mediated by DC-SIGN. J Virol 2007;81:13723–13734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martellini JA, Cole AL, Venkataraman N, et al. : Cationic polypeptides contribute to the anti-HIV-1 activity of human seminal plasma. FASEB J 2009;23:3609–3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stax MJ, van Montfort T, Sprenger RR, et al. : Mucin 6 in seminal plasma binds DC-SIGN and potently blocks dendritic cell mediated transfer of HIV-1 to CD4(+) T-lymphocytes. Virology 2009;391:203–211 [DOI] [PubMed] [Google Scholar]
- 9.O'Connor TJ, Kinchington D, Kangro HO, Jeffries DJ: The activity of candidate virucidal agents, low pH and genital secretions against HIV-1 in vitro. Int J STD AIDS 1995;6:267–272 [DOI] [PubMed] [Google Scholar]
- 10.Martellini JA, Cole AL, Svoboda P, et al. : HIV-1 enhancing effect of prostatic acid phosphatase peptides is reduced in human seminal plasma. PLoS One 2011;6:e16285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munch J, Rucker E, Standker L, et al. : Semen-derived amyloid fibrils drastically enhance HIV infection. Cell 2007;131:1059–1071 [DOI] [PubMed] [Google Scholar]
- 12.Roan NR, Liu H, Usmani SM, et al. : Liquefaction of semen generates and later degrades a conserved semenogelin peptide that enhances HIV infection. J Virol 2014;88:7221–7234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roan NR, Muller JA, Liu H, et al. : Peptides released by physiological cleavage of semen coagulum proteins form amyloids that enhance HIV infection. Cell Host Microbe 2011;10:541–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roan NR, Munch J, Arhel N, et al. : The cationic properties of SEVI underlie its ability to enhance human immunodeficiency virus infection. J Virol 2009;83:73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Ren R, Tan S, et al. : A peptide derived from the HIV-1 gp120 coreceptor-binding region promotes formation of PAP248–286 amyloid fibrils to enhance HIV-1 infection. PLoS One 2015;10:e0144522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zirafi O, Kim KA, Roan NR, et al. : Semen enhances HIV infectivity and impairs the antiviral efficacy of microbicides. Sci Transl Med 2014;6:262ra157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munch J, Sauermann U, Yolamanova M, Raue K, Stahl-Hennig C, Kirchhoff F: Effect of semen and seminal amyloid on vaginal transmission of simian immunodeficiency virus. Retrovirology 2013;10:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen SA, Carias AM, Anderson MR, et al. : Characterization of the influence of semen-derived enhancer of virus infection on the interaction of HIV-1 with female reproductive tract tissues. J Virol 2015;89:5569–5580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baggaley RF, White RG, Boily MC: HIV transmission risk through anal intercourse: Systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol 2010;39:1048–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Announcement: Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:458. [DOI] [PubMed] [Google Scholar]
- 21.Usmani SM, Zirafi O, Muller JA, et al. : Direct visualization of HIV-enhancing endogenous amyloid fibrils in human semen. Nat Commun 2014;5:3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnold F, Schnell J, Zirafi O, et al. : Naturally occurring fragments from two distinct regions of the prostatic acid phosphatase form amyloidogenic enhancers of HIV infection. J Virol 2012;86:1244–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott YM, Park SY, Dezzutti CS: Broadly neutralizing anti-HIV antibodies prevent HIV infection of mucosal tissue ex vivo. Antimicrob Agents Chemother 2016;60:904–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharkey DJ, Macpherson AM, Tremellen KP, Robertson SA: Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol Hum Reprod 2007;13:491–501 [DOI] [PubMed] [Google Scholar]
- 25.Introini A, Bostrom S, Bradley F, et al. : Correction: Seminal plasma induces inflammation and enhances HIV-1 replication in human cervical tissue explants. PLoS Pathog 2017;13:e1006492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anton PA, Cranston RD, Kashuba A, et al. : RMP-02/MTN-006: A phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses 2012;28:1412–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dublbecco R. and Ginsberg HS: End-point method measurement of the infectious titer of a viral sample. Virology: The Nature of Viruses, 2nd ed. Philadelphia, PA: J.P. Lippincott; 1988 [Google Scholar]
- 28.Fletcher PS, Elliott J, Grivel JC, et al. : Ex vivo culture of human colorectal tissue for the evaluation of candidate microbicides. AIDS 2006;20:1237–1245 [DOI] [PubMed] [Google Scholar]
- 29.Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA: Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol 2012;188:2445–2454 [DOI] [PubMed] [Google Scholar]
- 30.Herrera C, Cranage M, McGowan I, Anton P, Shattock RJ: Colorectal microbicide design: Triple combinations of reverse transcriptase inhibitors are optimal against HIV-1 in tissue explants. AIDS 2011;25:1971–1979 [DOI] [PubMed] [Google Scholar]
- 31.Richardson-Harman N, Parody R, Anton P, et al. : Analytical advances in the ex vivo challenge efficacy assay. AIDS Res Hum Retroviruses 2017;33:395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anton PA, Saunders T, Elliott J, et al. : First phase 1 double-blind, placebo-controlled, randomized rectal microbicide trial using UC781 gel with a novel index of ex vivo efficacy. PLoS One 2011;6:e23243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pilcher CD, Joaki G, Hoffman IF, et al. : Amplified transmission of HIV-1: Comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS 2007;21:1723–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dezzutti CS, Russo J, Wang L, et al. : Development of HIV-1 rectal-specific microbicides and colonic tissue evaluation. PLoS One 2014;9:e102585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stax MJ, Mouser EE, van Montfort T, et al. : Colorectal mucus binds DC-SIGN and inhibits HIV-1 trans-infection of CD4+ T-lymphocytes. PLoS One 2015;10:e0122020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grivel JC, Elliott J, Lisco A, et al. : HIV-1 pathogenesis differs in rectosigmoid and tonsillar tissues infected ex vivo with CCR5- and CXCR4-tropic HIV-1. AIDS 2007;21:1263–1272 [DOI] [PubMed] [Google Scholar]
- 37.Patyka M, Malamud D, Weissman D, Abrams WR, Kurago Z: Periluminal distribution of HIV-binding target cells and Gp340 in the oral, cervical and sigmoid/rectal mucosae: A mapping study. PLoS One 2015;10:e0132942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janocko L, Althouse AD, Brand RM, Cranston RD, McGowan I: The molecular characterization of intestinal explant HIV infection using polymerase chain reaction-based techniques. AIDS Res Hum Retroviruses 2015;31:981–991 [DOI] [PMC free article] [PubMed] [Google Scholar]