Figure 6. Mitochondrial Top3α Is Independent of the BTR Complex.
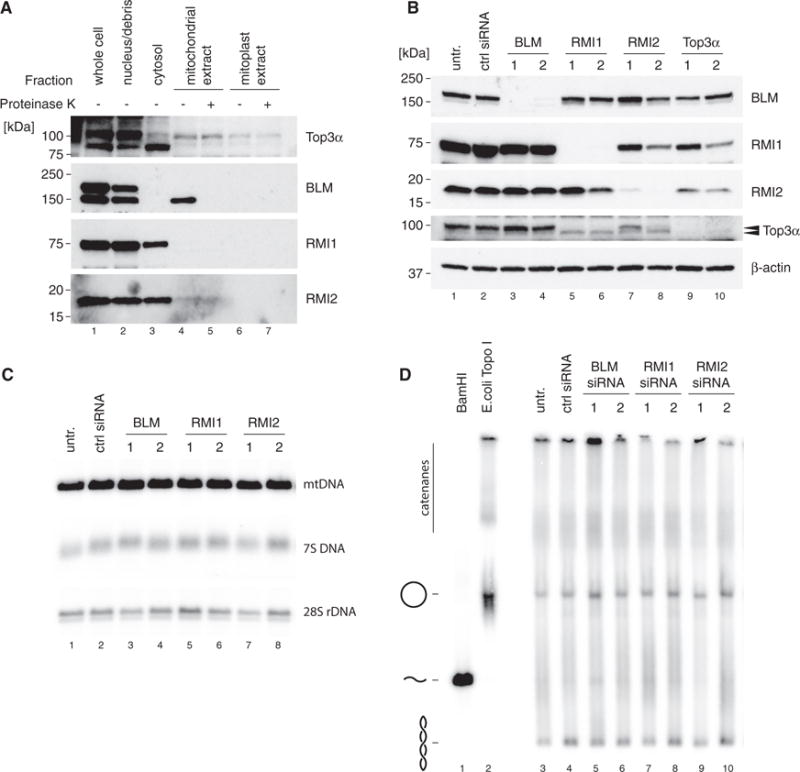
(A) Mitochondrial localization of protein components of the BTR complex by western blotting of cell fractions as in Figure 1A. Top3α blot is reproduced from Figure 1A for reference.
(B) Efficiency of depletion of BTR complex components assessed by western blotting of HeLa cell lysates. β-actin is used as a loading control. Arrows indicate the two forms or Top3α (nuclear and mitochondrial).
(C) Southern blot analysis of mtDNA copy number and 7S DNA levels following siRNA depletion of BLM, RMI1, and RMI2 as in (B). 28S rDNA is used as a loading control.
(D) mtDNA topology following siRNA depletion of BTR complex proteins. Uncut DNA (3 mg) was separated on low-percentage agarose, blotted, and detected using probe (a). BamHI-treated DNA marks the migration of linear mtDNA and E. coli TopoI-treated DNA marks the migration of relaxed, open circular form mtDNA.
