Abstract
Transcatheter aortic valve replacement (TAVR) has exploded into medical care for aortic stenosis, thus changing the treatment options for patients. TAVR is currently approved for extreme-risk, high-risk, and intermediate-risk patients with symptomatic severe aortic stenosis, and randomized trials for low-risk patients are underway. This article traces the trajectory of TAVR as a viable option for higher-risk patients and examines current outcomes.
Keywords: transcatheter aortic valve replacement, TAVR, surgical aortic valve replacement, SAVR, expandable valve
INTRODUCTION
Aortic stenosis has a long latent period and then rapid progression with high mortality once symptoms appear.1 The absence of an effective medical therapy for symptomatic severe aortic stenosis led to a class I indication for aortic valve replacement (AVR) in both U.S. and European guidelines.2,3 Unfortunately, a study by Bach and colleagues in 2009 found that up to half of patients with symptomatic severe aortic stenosis were not offered surgical aortic valve replacement (SAVR) because they were not considered reasonable surgical candidates based on age, comorbidities, frailty, or other anatomic risks.4 Transcatheter aortic valve replacement (TAVR) was developed as a less-invasive alternative to SAVR to allow treatment in this higher-risk patient population. Since the first successful TAVR in 2002,5 more than 250,000 procedures have been done worldwide with a steadily decreasing patient risk profile. This manuscript examines the current risk strata being treated with TAVR as well as current outcomes.
EXTREME-RISK PATIENTS
The onset of symptoms in severe aortic stenosis heralds the usually rapid clinical deterioration and a high mortality seen in the original survival curves of Ross and Braunwald1 and confirmed by contemporary authors such as Otto.6 If operated upon, extreme-risk patients are considered to have a > 50% chance of death or permanent disability at 30 days. Balloon aortic valvuloplasty (BAV) was developed to address this group of patients who could not undergo SAVR. Although BAV could decrease the gradient and improve flow dynamics, it did not improve survival.
The Placement of AoRTic TraNscathetER Valves (PARTNER) B trial in nonoperative patients was the first and only trial to randomize TAVR using the balloon-expandable valve against best medical therapy, which could include BAV.7 The primary end point was all-cause death. At 1 year, there was a 20% survival advantage to TAVR,7 and this advantage persisted for the 5-year life of the trial.8 The Extreme Risk Study of the CoreValve® U.S. Pivotal Trial was started after the 1-year end point of the PARTNER B trial was reported; by then, it was no longer considered reasonable to randomize against medical therapy due to the large advantage of TAVR. The CoreValve extreme-risk trial was a nonrandomized registry comparing TAVR with the self-expanding valve against a performance goal based on the 95% lower margin of survival for the medical arm of PARTNER B and five contemporary BAV series.9 TAVR with the self-expanding valve easily exceeded this goal at both 1 and 2 years.9,10 The REpositionable Percutaneous Replacement of Stenotic Aortic Valve Through Implantation of Lotus Valve System (Reprise III) trial extreme-risk arm randomized TAVR with a mechanically expandable valve against the self-expanding valve in a 2:1 fashion. The trial has completed enrollment and results will be presented in 2017. The PARTNER and CoreValve results have led to FDA approval for TAVR for both the balloon-expandable and self-expanding valves, with data pending for the mechanically expandable valve.
HIGH-RISK PATIENTS
High-risk patients are considered by the heart team to have a 30-day surgical mortality of more than 10% but are still operative candidates. High-risk patients constitute about 8% of the population currently considered for SAVR.
The PARTNER A trial randomized patients at high risk for SAVR in a 1:1 fashion between TAVR with the balloon-expandable valve vs SAVR. At 30 days and 1 year, the primary trial end point of all-cause mortality for TAVR vs SAVR was similar in both groups (3.4% vs 6.5% [P = .07] and 24.2% vs 26.8% [P = .44]). Of concern in this trial was that the rate of all neurologic events at 30 days and 1 year for TAVR vs SAVR was statistically higher in the TAVR arm (5.5% vs 2.4 % [P = .04] and 8.3% vs 4.3% [P = .04]).11 The rates of renal failure, major bleeding, and new or worsening atrial fibrillation were all higher in the SAVR group. The 5-year results for this trial continue to show equivalent survival in both groups, although the difference in neurologic events is no longer present.12
The high-risk arm of the CoreValve U.S. Pivotal Trial randomized TAVR with the self-expanding valve vs SAVR in a 1:1 fashion with a primary end point of all-cause mortality.13 In addition, the investigators addressed the issue of increased neurologic events seen in the TAVR arm of the Partner A trial by careful prospective evaluation for neurologic events of all patients; each individual was evaluated by the National Institute of Health Stroke Scale (NIHSS) pre-procedure, at every follow-up point, and after any event. All-cause mortality at 1 year for TAVR compared to SAVR was 14.2% vs 19.1% for superiority of P = .04. This is the only randomized TAVR trial to show superior survival to SAVR for the primary powered end point.13 The 2-year all-cause mortality was 22.2% for TAVR vs 28.6% for SAVR for continued statistical superiority at P < .05.14 The 3-year all-cause mortality for TAVR vs SAVR was 32.9% vs 39.1% (P = .07), which lost statistical superiority due to declining numbers but maintained a difference of 5.2% survival advantage in favor of TAVR.15 Additionally, the stroke rates for TAVR vs SAVR at 1 year and 3 years were 4.3% vs 8.3% (P = .04) and 12.6% vs 19.0% (P = .03), respectively; at both time points, TAVR was statistically superior. Major adverse cardiovascular and cerebrovascular events (MACCE) and flow hemodynamics were statistically superior for TAVR over SAVR at all time points to latest follow-up at 3 years. However, in both the PARTNER A and CoreValve trials, paravalvular leak (PVL) was more common in TAVR than SAVR. As stated earlier, these trials led to FDA approval for use of the balloon-expandable and self-expanding valves in high-risk patients.
There are two more high-risk trials underway. The Reprise III trial randomizes the mechanically expandable valve against a commercially available (FDA-approved) valve in a 2:1 fashion; this study has completed enrollment, and results for the primary end point are expected in 2017. The other trial is the Portico Re-sheathable Transcatheter Aortic Valve System U.S. Investigational Device Exemption study (Portico IDE) for an intra-annular self-expanding valve. This valve is also randomized against a commercially available valve, and the study remains actively enrolling.
INTERMEDIATE-RISK PATIENTS
Intermediate-risk patients assessed by the heart team are considered to have an operative mortality between 3% and 10%. The intermediate group studied represents about 12% of the current U.S. SAVR population.
The PARTNER II A trial randomized more than 2,000 patients between SAVR and TAVR using the second-generation balloon-expandable valve16 and with a primary end point of all-cause mortality or disabling stroke at 2 years. The mean Society of Thoracic Surgeons Predicted Risk of Mortality (STS PROM) was 5.8 % for both TAVR and SAVR. After 2 years, the Kaplan-Meier event rates for TAVR and SAVR were 19.3% vs 21.1% (P = .25) for noninferiority. It should be noted that if the TAVR-treated group only considered transfemoral TAVR (1,550/2,032, 76.3%), then the event rate compared to SAVR would be 16.3% vs 20% (P = .04) for superiority favoring TAVR. There was no difference in neurologic events at any time point. It is again noted that renal failure, major bleeding, and new or worsening atrial fibrillation was more common in the surgery group, whereas PVL was more common in the TAVR group but less so than it was in the original PARTNER A and CoreValve trials.
The third-generation balloon-expandable valve was tested in a nonrandomized observational study that enrolled more than 1,000 patients and had a preplanned propensity-matched analysis vs the SAVR arm of PARTNER II A.17 The mean STS PROM for the TAVR arm and the propensity-matched PARTNER II A arm were 5.2% and 5.4%. The all-cause mortality for the TAVR arm vs the matched SAVR arm were 1.1% vs. 4.0% (30 days) and 7.4% vs 13.0% (1 year). Stroke occurred in 2.7% of the TAVR arm and 6.1% of the SAVR arm. Despite the questions raised by propensity matching against a noncontemporaneous cohort, the very low observed-to-expected death ratio of 0.2 for the third-generation balloon-expandable valve is noteworthy. This data led to FDA approval of the balloon-expandable valve for intermediate-risk patients.
The SUrgical Replacement and Transcatheter Aortic Valve Implantation (SurTAVI) trial is a prospective randomized trial between the self-expanding TAVR valve and SAVR. The trial completed enrollment with more than 1,600 patients. The data, presented at the American College of Cardiology meeting in March 2017 and submitted to the FDA, found TAVR to be noninferior to SAVR for the primary composite end point of all-cause mortality and disabling stroke at 2 years in patients with symptomatic severe aortic stenosis.18 An intermediate-risk trial, Reprise IV, has been submitted to the FDA and will randomize TAVR using the mechanically expanded valve vs a commercially available balloon-expandable valve.
LOW RISK
Low-risk patients are determined by the heart team to have less than a 3% surgical risk or an STS PROM score of less than 4%. Two low-risk prospective randomized trials are currently enrolling in the United States: the PARTNER III trial of the balloon-expandable valve and the Evolut R low-risk randomized trial for the self-expanding valve.
CURRENT ISSUES
In the groups tested, TAVR has shown that it can provide survival, stroke risk, and hemodynamics equal to or better than SAVR. Additionally, both TAVR and SAVR lead to large and equivalent increases in quality of life as measured by a summary Kansas City Cardiomyopathy Quality of Life Score. The remaining issues center on PVL and valve durability, although pacemaker need in younger patients is also a concern.
Paravalvular Leak
Paravalvular leak occurs when the sealing area of the valve cannot adequately oppose the adjacent tissue of the patient's annular area. Significant PVL is a marker of poor long-term outcomes for both balloon-expandable and self/mechanical-expanding valves.13,19,20 There are commonly three basic causes of PVL: undersizing,21,22 malposition,23 and calcified annulus/left ventricular outflow tract.24,25
In the early TAVR experience, the annular size was gauged using 2-dimensional (2D) echocardiography.7 However, because the annulus is not round and the angle of the echo beam can be off axis, the annulus can be improperly measured, thus leading to undersizing. With increasing experience, TAVR operators have nearly universally shifted to 3D imaging to properly size the valve to the annulus.21,22 Computed tomography angiography is the most common modality chosen, but 3D echocardiography and cardiovascular magnetic resonance imaging have also been used. Since the use of these modalities leads to proper aortic annulus sizing, the undersizing pitfall has been largely eliminated.
During deployment, TAVR prosthesis placement is crucial. If the prosthesis is implanted too high (too aortic) or too low (too ventricular), significant PVL may occur. The self-expanding and mechanically expandable valves all have the ability to recapture, reposition, and redeploy, allowing more than one attempt to achieve an ideal valve position.
The third common reason for PVL is patient anatomy, such as bulky calcium that prevents adequate expansion of the valve frame against the adjoining tissue. Advancements in TAVR technology have led to novel means to combat this issue. For instance, sealing skirts have been added to the latest iterations of these valves to further decrease the incidence of PVL.13,26,27 These advances have allowed the PVL rates in TAVR to begin to approach the results we see in surgery (Figure 1).7,9,11,13,28–30
Figure 1.
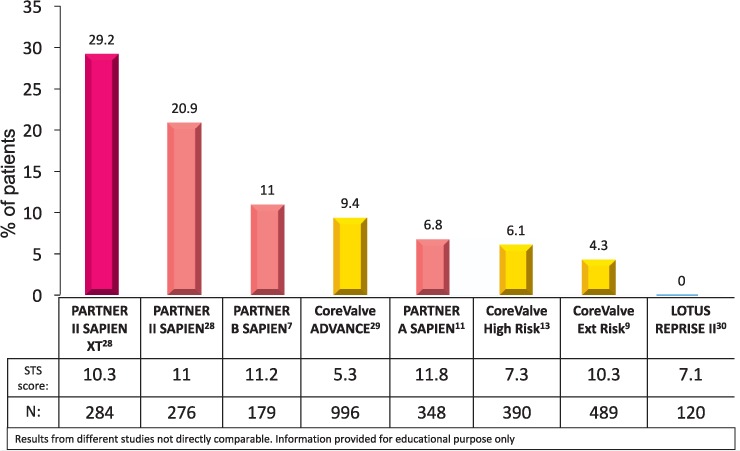
The decrease in 12-month moderate-to-severe paravalvular leak in transcatheter aortic valve replacement in less than a decade.7,9,11,13,28–30
Durability
Patients who receive SAVR have two basic choices for their valve type: mechanical or tissue. Mechanical valves have greater long-term durability and lower reoperation rates; however, they require long-term anticoagulation. Alternatively, tissue valves do not require indefinite anticoagulation and thus carry a lower bleeding risk. Durability is the main disadvantage of bioprosthetic valves since they are subject to fibrocalcification and degeneration over time.
Patient age has traditionally been the first decision point in selecting a mechanical vs tissue valve; thus, informed prosthetic valve selection for middle-aged patients requires an in-depth discussion. Other issues to consider are refusal of or contraindication to full anticoagulation.
The long-term survival for open AVR patients over 50 years old is the same for mechanical and bioprosthetic valves.31 Furthermore, recent data suggest that despite an increased risk of reoperation, patients under the age of 60 who select a bioprosthetic valve do not trade off long-term survival when compared with patients who select a mechanical valve.32,33
We do know two facts that apply as patients age. The first is that for each year you live, your remaining expected life span is shorter. We also know that for tissue valves, the older you are when the valve is placed, the slower it will degenerate—in fact, for valves placed after age 70, valve degeneration within the patient's lifetime is uncommon.34,35 This highlights a gap in current research: Since the mean age in the U.S. TAVR trials is greater than 80 years old, we are not likely to get good durability data unless TAVR valves fail early, which so far has not occurred.
The long-term durability of TAVR is still in question. Currently, the most complete long-term data with TAVR shows excellent results when compared to SAVR. Deeb et al. demonstrated not only that self-expanding TAVR in high-risk patients with severe aortic stenosis had superior 3-year clinical outcomes compared to SAVR, but also that TAVR valves showed more favorable hemodynamics.36
Longer-term follow-up of the intermediate-risk trials and low-risk randomized trials, which include younger patients, will be the only way to establish the long-term durability of TAVR valves.
CONCLUSION
TAVR has changed the face of aortic stenosis treatment. Based on the data trends so far and extensive worldwide experience, we expect that TAVR will demonstrate noninferiority to SAVR in low-risk patients. Furthermore, if trials demonstrate TAVR's long-term durability, it will become the treatment of choice for symptomatic severe aortic stenosis in all patients anatomically suitable for TAVR who are candidates for a biologic valve.
KEY POINTS
TAVR is approved for extreme-risk, high-risk, and intermediate-risk patients with symptomatic severe aortic stenosis.
Low-risk randomized trials are currently underway for potential FDA approval.
TAVR has equivalent or superior survival for high-risk and intermediate-risk patients compared to SAVR.
Footnotes
Conflict of Interest Disclosure:
The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
REFERENCES
- 1. Ross J Jr, Braunwald E.. Aortic stenosis. Circulation. 1968. July; 38 1 Suppl: 61– 7. [DOI] [PubMed] [Google Scholar]
- 2. Nishimura RA, Otto CM, Bonow RO, . et al . ;American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014. June 10; 63 22: e57– 185. [DOI] [PubMed] [Google Scholar]
- 3. Vahanian A, Alfieri O, Andreotti F, . et al. [Guidelines on the management of valvular heart disease (version 2012). The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)]. G Ital Cardiol (Rome). 2013. March; 14 3: 167– 214. [DOI] [PubMed] [Google Scholar]
- 4. Bach DS, Siao D, Girard SE, Duvernoy C, McCallister BD Jr, Gualano SK.. Evaluation of patients with severe symptomatic aortic stenosis who do not undergo aortic valve replacement: the potential role of subjectively overestimated operative risk. Circ Cardiovasc Qual Outcomes. 2009. November; 2 6: 533– 9. [DOI] [PubMed] [Google Scholar]
- 5. Cribier A, Eltchaninoff H, Bash A, . et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002. December 10; 106 24: 3006– 8. [DOI] [PubMed] [Google Scholar]
- 6. Otto CM, Pearlman AS, Gardner CL.. Hemodynamic progression of aortic stenosis in adults assessed by Doppler echocardiography. J Am Coll Cardiol. 1989. March 1; 13 3: 545– 50. [DOI] [PubMed] [Google Scholar]
- 7. Leon MB, Smith CR, Mack M, . et al .; PARTNER Trial Investigators Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010. October 21 363 17: 1597– 607. [DOI] [PubMed] [Google Scholar]
- 8. Kapadia SR, Tuzcu EM, Makkar RR, . et al. Long-term outcomes of inoperable patients with aortic stenosis randomly assigned to transcatheter aortic valve replacement or standard therapy. Circulation. 2014. October 21; 130 17: 1483– 92. [DOI] [PubMed] [Google Scholar]
- 9. Popma JJ, Adams DH, Reardon MJ, . et al .; CoreValve United States Clinical Investigators Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014. May 20; 63 19: 1972– 81. [DOI] [PubMed] [Google Scholar]
- 10. Yakubov SJ, Adams DH, Watson DR, . et al .; CoreValve United States Clinical Investigators 2-Year outcomes after iliofemoral self-expanding transcatheter aortic valve replacement in patients with severe aortic stenosis deemed extreme risk for surgery. J Am Coll Cardiol. 2015. September 22; 66 12: 1327– 34. [DOI] [PubMed] [Google Scholar]
- 11. Smith CR, Leon MB, Mack MJ, . et al .; PARTNER Trial Investigators Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011. June 9; 364 23: 2187– 98. [DOI] [PubMed] [Google Scholar]
- 12. Mack MJ, Leon MB, Smith CR, . et al .; PARTNER 1 Trial Investigators 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015. June 20; 385 9986: 2477– 84. [DOI] [PubMed] [Google Scholar]
- 13. Adams DH, Popma JJ, Reardon MJ, . et al .; U.S. CoreValve Clinical Investigators Transcatheter aortic-valve replacement with a selfexpanding prosthesis. N Engl J Med. 2014. May 8; 370 19: 1790– 8. [DOI] [PubMed] [Google Scholar]
- 14. Reardon MJ, Adams DH, Kleiman NS, . et al. 2-Year Outcomes in Patients Undergoing Surgical or Self-Expanding Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2015. July 14; 66 2: 113– 21. [DOI] [PubMed] [Google Scholar]
- 15. Deeb GM, Reardon MJ, Chetcuti S, . et al .; CoreValve US Clinical Investigators 3-Year Outcomes in High-Risk Patients Who Underwent Surgical or Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2016. June 7; 67 22: 2565– 74. [DOI] [PubMed] [Google Scholar]
- 16. Leon MB, Smith CR, Mack MJ, . et al .; PARTNER 2 Investigators Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016. April 28; 374 17: 1609– 20. [DOI] [PubMed] [Google Scholar]
- 17. Thourani VH, Kodali S, Makkar RR, . et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016. May 28; 387 10034: 2218– 25. [DOI] [PubMed] [Google Scholar]
- 18. Reardon MJ, Van Mieghem NM, Popma JJ, . et al .; SURTAVI Investigators surgical or transcatheter aortic-valve replacement in intermediate-risk patients. New Engl J Med. 2017. April 6; 376 14: 1321– 31. [DOI] [PubMed] [Google Scholar]
- 19. Gotzmann M, Korten M, Bojara W, . et al. Long-term outcome of patients with moderate and severe prosthetic aortic valve regurgitation after transcatheter aortic valve implantation. Am J Cardiol. 2012. November 15; 110 10: 1500– 6. [DOI] [PubMed] [Google Scholar]
- 20. Gilard M, Eltchaninoff H, Iung B, . et al .; FRANCE 2 Investigators Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med. 2012. May 3; 366 18: 1705– 15. [DOI] [PubMed] [Google Scholar]
- 21. Willson AB, Webb JG, Labounty TM, . et al. 3-dimensional aortic annular assessment by multidetector computed tomography predicts moderate or severe paravalvular regurgitation after transcatheter aortic valve replacement: a multicenter retrospective analysis. J Am Coll Cardiol. 2012. April 3; 59 14: 1287– 94. [DOI] [PubMed] [Google Scholar]
- 22. Jilaihawi H, Kashif M, Fontana G, . et al. Cross-sectional computed tomographic assessment improves accuracy of aortic annular sizing for transcatheter aortic valve replacement and reduces the incidence of paravalvular aortic regurgitation. J Am Coll Cardiol. 2012. April 3; 59 14: 1275– 86. [DOI] [PubMed] [Google Scholar]
- 23. Sherif MA, Abdel-Wahab M, Stöcker B, . et al. Anatomic and procedural predictors of paravalvular aortic regurgitation after implantation of the Medtronic CoreValve bioprosthesis. J Am Coll Cardiol. 2010. November 9; 56 20: 1623– 9. [DOI] [PubMed] [Google Scholar]
- 24. Khalique OK, Hahn RT, Gada H, . et al. Quantity and location of aortic valve complex calcification predicts severity and location of paravalvular regurgitation and frequency of post-dilation after balloon-expandable transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2014. August; 7 8: 885– 94. [DOI] [PubMed] [Google Scholar]
- 25. Ewe SH, Ng AC, Schuijf JD, . et al. Location and severity of aortic valve calcium and implications for aortic regurgitation after transcatheter aortic valve implantation. Am J Cardiol. 2011. November 15; 108 10: 1470– 7. [DOI] [PubMed] [Google Scholar]
- 26. Kodali S, Thourani VH, White J, . et al. Early clinical and echocardiographic outcomes after SAPIEN 3 transcatheter aortic valve replacement in inoperable, high-risk and intermediate-risk patients with aortic stenosis. Eur Heart J. 2016. July 21; 37 28: 2252– 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meredith Am IT, Walters DL, Dumonteil N, . et al. Transcatheter aortic valve replacement for severe symptomatic aortic stenosis using a repositionable valve system: 30-day primary end point results from the REPRISE II study. J Am Coll Cardiol. 2014. September 30; 64 13: 1339– 48. [DOI] [PubMed] [Google Scholar]
- 28. Leon MB; PARTNER Trial Investigators. . A randomized evaluation of the SAPIEN XT Transcatheter Valve System in Patients with Aortic Stenosis Who Are Not Candidates for Surgery: PARTNER II, Inoperable Cohort. Paper presented at: ACC 2013; 2013 Mar 10; San Francisco, CA. [Google Scholar]
- 29. Linke A, Bosmans J, Gerckens U.. ADVANCE II: best practices investigation in patients undergoing transcatheter aortic valve implantation with a self-expanding valve. Paper presented at: EuroPCR 2014; 2014 May 20–23; Paris, France. [Google Scholar]
- 30. Meredith I. Pitfalls of TAVI: have they been resolved? Selecting the right patient for TAVI. Paper presented at: PCR London Valve; 2014 Sep 28–30; London, UK. [Google Scholar]
- 31. Khan SS, Trento A, DeRobertis M, . et al. Twenty-year comparison of tissue and mechanical valve replacement. J Thorac Cardiovasc Surg. 2001. August; 122 2: 257– 69. [DOI] [PubMed] [Google Scholar]
- 32. Ruel M, Chan V, Bédard P, . et al. Very long-term survival implications of heart valve replacement with tissue versus mechanical prostheses in adults <60 years of age. Circulation. 2007. September 11; 116 11 Suppl: 1294– 300. [DOI] [PubMed] [Google Scholar]
- 33. Chiang YP, Chikwe J, Moskowitz AJ, Itagaki S, Adams DH, Egorova NN, . et al. Survival and long-term outcomes following bioprosthetic vs mechanical aortic valve replacement in patients aged 50 to 69 years. JAMA. 2014. October 1; 312 13: 1323– 9. [DOI] [PubMed] [Google Scholar]
- 34. Jamieson WR, Munro AI, Miyagishima RT, Allen P, Burr LH, Tyers GF.. Carpentier-Edwards standard porcine bioprosthesis: clinical performance to seventeen years. Ann Thorac Surg. 1995. October; 60 4: 999– 1006; discussion 1007. [DOI] [PubMed] [Google Scholar]
- 35. Fann JI, Miller DC, Moore KA, . et al. Twenty-year clinical experience with porcine bioprostheses. Ann Thorac Surg. 1996. November; 62 5: 1301– 11; discussion 1311–2. [DOI] [PubMed] [Google Scholar]
- 36. Deeb GM, Reardon MJ, Chetcuti S, . et al. 3-year outcomes in high-risk patients who underwent surgical or transcatheter aortic valve replacement. J Am Coll Cardiol. 2016. June 7; 67 22: 2565– 74. [DOI] [PubMed] [Google Scholar]


