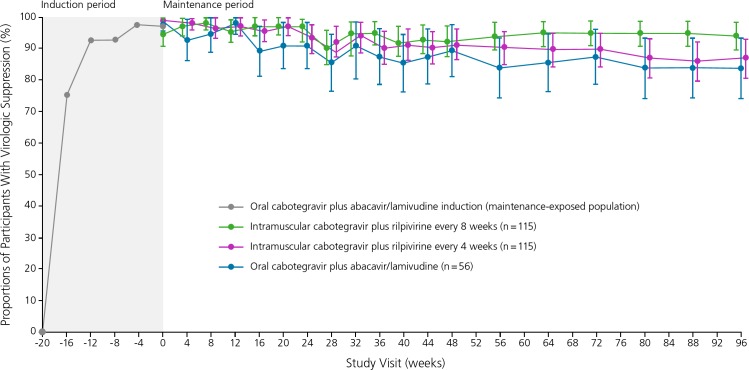
Figure 3.
Rates of virologic suppression among HIV-infected individuals treated with the investigational drug cabotegravir 30 mg plus coformulated (indicated with a /) abacavir/lamivudine orally for 20 weeks (induction phase), followed by continued cabotegravir 400 mg plus rilpivirine 600 mg intramuscularly every 4 weeks, cabotegravir 600 mg plus rilpivirine 900 mg intramuscularly every 8 weeks, or cabotegravir 30 mg plus abacavir/lamivudine orally daily (maintenance therapy) in the phase II LATTE-2 (Long-Acting Antiretroviral Treatment Enabling-2) trial. Adapted from Margolis et al.24