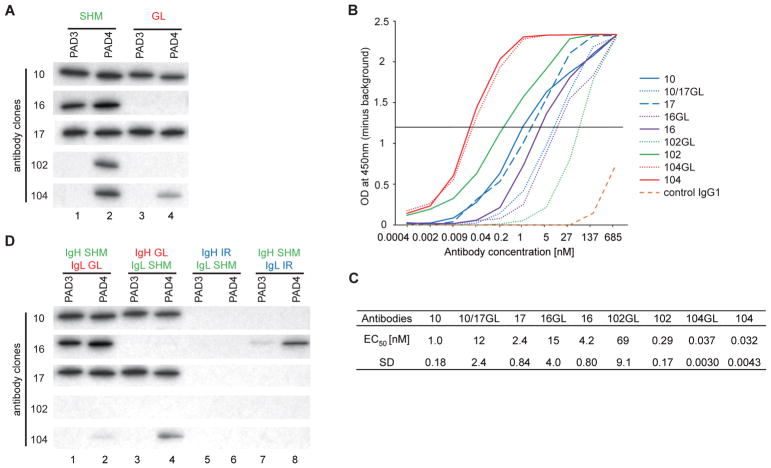
Fig. 2.
The importance of SHM in antigen recognition by human monoclonal antibodies to PAD4. (A) Antibody binding to PAD3/PAD4 was addressed by IP of radiolabeled substrates using mutated (SHM) and germline (GL) reverted anti-PAD4 antibodies (3.4 nM antibody/assay). Antibodies 10GL and 17GL are the same antibody (10/17GL). (B) Anti-PAD4 antibodies and their germline variants were titrated from 0.4 pM to 685 nM against purified PAD4 by ELISA. (C) Half maximal effective concentration (EC50) for anti-PAD4 binding were calculated from B (SD, standard deviation). (D) The heavy and light chain (IgH and IgL, respectively) of SHM and GL reverted anti-PAD4 antibodies were combined to generate monoclonal antibodies (lanes 1–4). Irrelevant (IR) IgH and IgL from an antibody with no reactivity to PADs were used to produce anti-PAD4 hybrid antibodies (lanes 5–8). Antibody binding to PAD3/PAD4 was addressed by IP (3.4 nM antibody/assay) using radiolabeled substrates. The experiments in A and D were performed on two separate occasions with similar results.