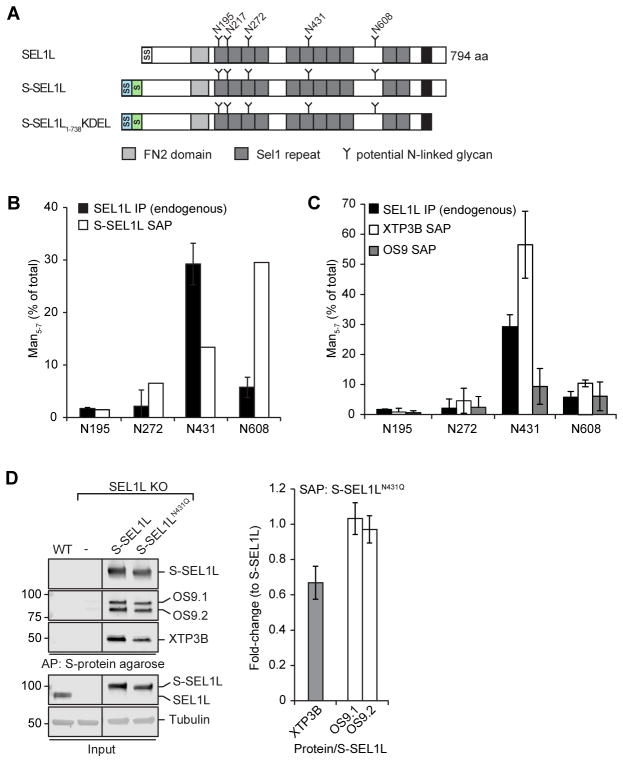
Figure 6. XTP3B binds preferentially to SEL1L bearing a demannosylated glycan at Asn431.
(A) Schematic representation of the domain organization of SEL1L and a full-length and luminal S-tagged SEL1L construct. SS=Signal Sequence; S=S-peptide tag; TM=transmembrane domain. (B, C) Fraction of SEL1L tryptic N-glycopeptides bearing trimmed (Man5-7) glycans at the indicated sequons. Quantification of tryptic glycopeptides from endogenous SEL1L (B, C; n=2) immunoprecipitated from HEK293 cells or S-affinity purified (SAP) from cells expressing S--SEL1L (B; n=1), S-OS9.2 (C; n=2) or XTP3B-S (C; n=6) from transfected HEK293 cells. Error bars represent STDEV. Endogenous SEL1L IP data in B and C are the same. (D) S-SEL1L was affinity purified from 1% Triton X-100 lysates of SEL1L KO cells transfected with S-SEL1L, S-SEL1LN431Q, or empty vector. SEL1L complexes were analyzed by immunoblotting with the indicated antibodies. Quantification of XTP3B and OS9 was normalized to the amount of SEL1L precipitated and represented as mean±STDEV (n=3 for XTP3B; n=2 for OS9). Lanes in which transient S-SEL1L expression most closely reflected endogenous SEL1L levels were selected, lanes representing lower expression were removed. See also Fig. S5.