Abstract
The vast majority of maintenance dialysis patients suffer from poor long-term survival rates and lower levels of health-related quality of life. However, home hemodialysis is a historically significant dialysis modality that has been associated with favorable outcomes as well as greater patient autonomy and control, yet only represents a small minority of the total dialysis performed in the United States. Some potential disadvantages of home hemodialysis include vascular access complications, infection-related hospitalizations, patient fatigue and attrition. In addition, current barriers and challenges in expanding the utilization of this modality include limited patient and provider education and technical expertise. Here we report a 65-year old male with end-stage renal disease due to Alport’s Syndrome who has undergone 35 years of uninterrupted thrice-weekly home hemodialysis (i.e., every Sunday, Tuesday, and Thursday evening, each session lasting 3 to 3¼ hours in length) using a conventional hemodialysis machine who has maintained a high functional status allowing him to work six to eight hours per day. The patient has been able to liberalize his dietary and fluid intake while only requiring three to four liters of ultrafiltration per treatment, despite having absence of residual kidney function. Through this case of extraordinary longevity and outcomes after 35 years of dialysis and a review of the literature, we illustrate the history of home hemodialysis, its significant clinical and psychosocial advantages, as well as the barriers that hinder its widespread adaptation.
Keywords: End stage renal disease, home hemodialysis, longevity
INTRODUCTION
Home hemodialysis represents a small but increasing proportion of dialysis treatments utilized in the United States. In this case report, we describe a patient who receives home hemodialysis on a thrice-weekly basis with an exceptional dialysis vintage exceeding 35 years. In addition, a brief overview of the history of home hemodialysis and its current epidemiology, outcomes data, clinical advantages and limitations, and future research directions of this renal replacement modality is provided.
CASE
The patient is a 65-year old Asian American male with a history significant for end stage renal disease secondary to Alport’s Syndrome. His family history is notable for Alport’s syndrome in his sister as well as both of his daughters. The patient was first diagnosed with chronic kidney disease with heavy proteinuria and hematuria at age 18 (Figure 1), and he underwent a kidney biopsy at age 19 confirming the diagnosis of Alport’s Syndrome. The patient’s kidney disease gradually progressed over time, and by the age of 28 he initiated in-center thrice-weekly hemodialysis. After three months of in-center hemodialysis, he then transitioned to thrice-weekly home hemodialysis. The patient’s wife received formal hemodialysis training and was primarily responsible for assisting him with completion of his dialysis treatments. Due to limitations in the efficiency of his dialysis treatment related to antiquated dialysis membranes and lack of high-flux dialyzers, he was initially prescribed four-hour dialysis treatments three times per week to achieve adequate clearance. However, as high-flux dialyzers became widely available, his dialysis duration was shortened to approximately three hours to three hours and fifteen minutes per treatment. The patient was educated about peritoneal dialysis as a potential alternative dialysis modality, but given his concerns about the potential complications (e.g., peritoneal infections), he opted for hemodialysis instead.
Figure 1.
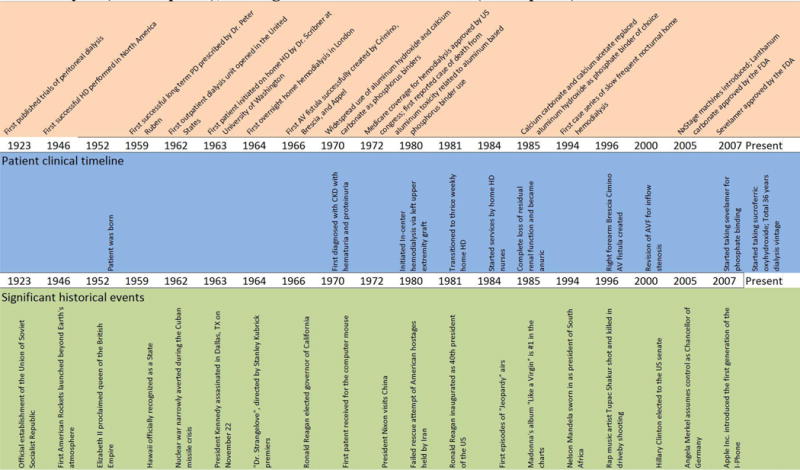
Timeline of history of home hemodialysis (upper panel), patient’s clinical course on home hemodialysis (middle panel), and significant historical events (lower panel).
Since the age of 29, the patient has had a long series of dialysis accesses. His first vascular access was a left upper extremity arteriovenous graft (AVG) with five subsequent revisions to his AVG with intermittent requirement of temporary tunneled dialysis catheters on multiple occasions. At the age of 45, the patient underwent construction of a right brachiocephalic arteriovenous fistula (AVF), which has remained functional and has continued to serve as his primary vascular access until the present day.
The patient continued to have residual kidney function for the first five years following initiation of hemodialysis, but he became anuric by the age of 35. The patient was previously evaluated for kidney transplantation, but declined this intervention due to work obligations and inability to take time off. In addition, the patient was identified as having two vessel coronary disease with right coronary artery and left anterior descending arterial involvement (Figure 2), as well as a left ventricular systolic function of 52% with mild to moderate aortic regurgitation and moderately elevated pulmonary artery systolic pressure shown on most recent echocardiogram.
Figure 2.

Chest computed tomography scan with calcium scoring. Note heavily calcified coronary vessels.
His most recent chest radiography showed a small chronic right pleural effusion as well as Rugger-Jersey sign synonymous with hyperparathyroidism (Figure 3) in the context of an intact parathyroid hormone level of 731 pg/mL, corrected serum calcium of 8.7 mg/dL, and serum phosphorus of 6.3 mg/dL. His current chronic kidney disease mineral bone disorder medication regimen includes paricalcitol 2 mcg daily and cinacalcet 30 mg daily. His phosphorus binder regimen consists of sucroferric oxyhydroxide 1 tablet with meals, and sevelamer 3200 mg with meals. With respect to his anemia management, he is currently receiving intravenous iron sucrose 100 mg monthly and methoxy polyethylene glycol-epoetin beta (Mircera) 50 mcg every 2 weeks.
Figure 3.

Chest radiography illustrating severe renal osteodystrophy and Rugger-Jersey spine.
With respect to his nutritional status, the patient has been able to consume a liberal diet. He has not closely enumerated phosphorus or protein contents in his diet but has generally avoided foods that are replete with phosphorus and potassium. Most of his meals are prepared at home, consisting primarily of “simple Chinese foods” (e.g., white rice, stir fried vegetables, select meats). He endorses eating out at restaurants two to three times per week. The patient denies any difficulties in maintaining his appetite and has had to restrict total dietary intake to prevent excessive weight gain.
Using his own financial resources, the patient was able to install an adequate plumbing and water purification into his private residence to accommodate the usage of a conventional hemodialysis machine. In addition, he was able to acquire the services of in-home dialysis nursing staff to aid in the cannulation of his arteriovenous fistula and execution of dialysis. Since the advent of high-flux dialyzers, the patient has consistently adhered to a thrice-weekly dialysis schedule with session lengths of no more than three hours and fifteen minutes due to fatigue with longer treatment durations. If his dialysis adequacy as ascertained by his single-pool Kt/V falls below target, the flexibility of home hemodialysis allows him to increase his treatment frequency to four times per week. Average ultrafiltration volume per dialysis treatment has typically ranged between three to four liters. In terms of his treatment schedule, each hemodialysis session is usually initiated at six PM and ends between nine to ten PM, after which time the patient usually stays awake for an additional two to three hours prior to sleeping.
The flexibility of home hemodialysis has allowed the patient to maintain an active lifestyle. For example, he is the owner of a biotechnology company which requires frequent travel, and he visits international destinations at least annually. Furthermore, he has maintained a high functional status and regularly attends ballroom dance lessons with his wife twice per week. Notably, the patient has a strong belief in the efficacy of traditional Eastern medications and specifically musculoskeletal manipulation of reflexology as hypothesized to relate to the optimal function of individual organ systems. He performs foot, hand, and body massages daily, which he credits as maintaining optimal control of serum calcium and phosphorus. He does not, however, partake in herbal supplementation.
While there are multiple aspects that have contributed to this patient’s exceptional longevity (i.e., ample social support, adequate nutritional intake, principal use of an AVF/AVG), the overarching predominant factor has been the patient’s ability to undergo dialysis in the home setting. The heightened autonomy, flexibility, and control that the patient has been able to experience through the receipt of home hemodialysis has also provided him with greater empowerment to be self-directed and wholly engaged in his care, while also allowing him to maintain an active lifestyle and a high level of socialization and independence. Hence, the remainder of this review will focus upon home hemodialysis as a modality that has the potential to optimize patient survival and health-related quality of life.
DISCUSSION
History of Home Hemodialysis
Home hemodialysis was once a widely utilized renal replacement modality first implemented in 1964 by three groups located broadly in Seattle, Boston, and London with the first report of overnight unattended hemodialysis described in the United Kingdom in 1965.1 Early hemodialysis treatments were largely conducted in a home setting due to a lack of hospital and clinic based facilities. In 1973, home hemodialysis was used by nearly 40% of all dialysis patients in the United States, and the United States Congress passed social security amendments that created a Medicare program to pay for dialysis treatment, incentivizing the building of dialysis units and the rising utilization of in-center hemodialysis2. The advent of peritoneal dialysis in the 1970’s and 1980’s provided further alternatives for home hemodialysis, reducing its popularity.2 By the early 2000’s, only Australia, New Zealand, and Turkey had substantial numbers of patients on home hemodialysis3. In 1994, slow, frequent, nocturnal home hemodialysis was first implemented in a Canadian center4. Advancements in dialysis technology over the past two decades have led to greater access to home hemodialysis. Currently multiple dialysis machines have been commissioned for home hemodialysis including the Aksys PHD machine (Aksys Ltd., Lincolnshire, IL), NxStage System One (NxStage Medical, Inc., Lawrence, MA), and the Fresenius 2008K@HOME (Fresenius USA Inc., Walnut Creek, CA).5 Due to its relatively small size, low flow fraction and thus low volume dialysate use, and lack of requirement for home water treatment system installation, NxStage System One has been the most commonly employed system in the United States to date.
Epidemiology of Home Hemodialysis
Home hemodialysis represents a small but increasing fraction of renal replacement therapy utilized by both incident and prevalent end stage renal disease patients in the United States. Representing <1% of total dialysis in 20052, the incidence of home hemodialysis has risen by 120% over the period of 2007 to 2014, but is still only utilized by 3.4% of all incident hemodialysis patients. Among the prevalent end stage renal disease population, total home dialysis (including both peritoneal dialysis and home hemodialysis) increased from 8.7% in 2008 to 11.6% in 2014.6 Among patients treated with home dialysis, the proportion of those using home hemodialysis rose from 6.2% in 2000 to 15.6% in 2014, a 2.5 fold increase6.
Home Hemodialysis Outcomes
Mortality
In the Frequent Hemodialysis Network trial, 52 end stage renal disease patients were randomized to undergo thrice weekly in-center hemodialysis vs. six times weekly nocturnal hemodialysis. Although the trial was not sufficiently powered to show differences in mortality, patients in the frequent nocturnal hemodialysis group were noted to have greater improvement in mean left ventricular mass7. While this trial focused upon in-center hemodialysis patients, the favorable effects of more frequent hemodialysis may also be observed with home hemodialysis, particularly given the flexibility in treatment schedules of home-based dialysis therapies.
Risk factors associated with increased mortality in home hemodialysis patients include increasing age, diabetes, and smoking.8 In a large retrospective analysis of 2840 incident home hemodialysis patients, one-year mortality for patients on home hemodialysis was 7.6%, with 24.9% of patients undergoing discontinuation of home hemodialysis during this time period. In this study, factors associated with discontinuation of home hemodialysis included diabetes, tobacco use, alcohol, and recreational drug use9 In comparison to peritoneal dialysis, home hemodialysis has been associated with a 20% lower risk of all-cause mortality, and 30% reduction in risk for technique failure.10 Home hemodialysis, however, has not shown mortality benefits when compared to kidney transplantation. A prospective study of 480 incident US home hemodialysis and 480 matched incident kidney transplant patients showed that, as compared to kidney transplant, home hemodialysis has been associated with a four-fold higher risk of death.11
Patient centered outcomes
Data from a large observational study comparing six-times per week home hemodialysis vs. thrice weekly in-center hemodialysis are also available and have shown greater improvement in health-related quality of life, reductions in depression, and decreases in post-dialysis recovery time with the former modaliy.12 Furthermore, nocturnal home hemodialysis offers a significant psycho-social advantage to conventional in-center hemodialysis including less disruption of daytime schedules, less travel to clinic, better quality of sleep, greater time allotment for work and time spent with family, as well as improved sexual function.13
Complications
Home hemodialysis may also be associated with some complications. In a randomized control trial of 87 hemodialysis patients randomized to conventional thrice-weekly in-center hemodialysis vs. six days per week nocturnal hemodialysis, a trend towards higher risk of need for access repair, access loss, or access hospitalization was observed in patients undergoing nocturnal hemodialysis14. In addition, home hemodialysis has been associated with higher rates of hospitalization due to vascular access infections in comparison to conventional in-center hemodialysis.15 Among home hemodialysis patients, the use of central venous catheters was associated with the highest rates of hospitalization.16
Future Research Directions
Despite the advantages of home hemodialysis, significant barriers exist in its widespread implementation including lack of adequate resources for patient education and training, limited provider experience, technical complexities of operating a dialysis machine, and greater water purification requirements.17 At this time, current knowledge gaps and future research directions relating to home hemodialysis include 1) the identification of patients who are optimal for this dialysis modality, 2) development of more streamlined and portable home hemodialysis technologies,18 3) improvements in home hemodialysis vascular access durability,2,19 4) greater incorporation of telemedicine for home therapies,20 and 5) the role of home hemodialysis in pediatric end stage renal disease patients.21 Further investigations are needed to demonstrate home hemodialysis as a robust modality of renal replacement therapy.
CONCLUSION
End stage renal disease patients receiving maintenance hemodialysis suffer from high mortality rates and restricted health-related quality of life6. In this case report, we delineated the experience of a patient with end stage renal disease on an unconventional yet successful thrice-weekly nocturnal home hemodialysis regimen. The patient had the added benefit of having the financial resources to allow for home water system modification, private nursing staff, and the use of a conventional hemodialysis machine in lieu of traditional home hemodialysis systems. Access to home hemodialysis has allowed the patient to maintain an active, highly functional lifestyle, with minimal restrictions to his vocational and personal responsibilities, while also achieving an unparalleled dialysis vintage, giving new meaning to the adage, “There’s no place like home.” Hence, further studies are needed to expand access of home hemodialysis as a feasible and successful dialysis modality across a broader population of patients.
Acknowledgments
Funding Support:
The authors are supported by the research grants from the American Thyroid Association (CMR) and National Kidney Foundation (CMR); NIH/NIDDK including K23-DK102903 (CMR), R03-DK114642 (CMR), and K24-DK091419 (KKZ); and philanthropist grants from Mr. Harold Simmons, Mr. Louis Chang, and Dr. Joseph Lee.
References
- 1.Baillod RA. Home dialysis: lessons in patient education. Patient Educ Couns. 1995;26:17–24. doi: 10.1016/0738-3991(95)00721-b. [DOI] [PubMed] [Google Scholar]
- 2.Trinh E, Chan CT. The Rise, Fall, and Resurgence of Home Hemodialysis. Semin Dial. 2017;30:174–80. doi: 10.1111/sdi.12572. [DOI] [PubMed] [Google Scholar]
- 3.Kerr PG, Jaw J. Home Hemodialysis: What Is Old Is New Again. Contrib Nephrol. 2017;190:146–55. doi: 10.1159/000468961. [DOI] [PubMed] [Google Scholar]
- 4.Uldall R, Ouwendyk M, Francoeur R, et al. Slow nocturnal home hemodialysis at the Wellesley Hospital. Adv Ren Replace Ther. 1996;3:133–6. doi: 10.1016/s1073-4449(96)80053-7. [DOI] [PubMed] [Google Scholar]
- 5.Moran J, Kraus M. Starting a home hemodialysis program. Semin Dial. 2007;20:35–9. doi: 10.1111/j.1525-139X.2007.00239.x. [DOI] [PubMed] [Google Scholar]
- 6.National Institutes of Health NIoDaDaKD, editor. System USRD. USRDS annual data report: Epidemiology of kidney disease in the United States. Bethesda: 2016. p. MD2016. [Google Scholar]
- 7.Culleton BF, Walsh M, Klarenbach SW, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA. 2007;298:1291–9. doi: 10.1001/jama.298.11.1291. [DOI] [PubMed] [Google Scholar]
- 8.Perl J, Na Y, Tennankore KK, Chan CT. Temporal Trends and Factors Associated with Home Hemodialysis Technique Survival in Canada. Clin J Am Soc Nephrol. 2017 doi: 10.2215/CJN.13271216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seshasai RK, Mitra N, Chaknos CM, et al. Factors Associated With Discontinuation of Home Hemodialysis. Am J Kidney Dis. 2016;67:629–37. doi: 10.1053/j.ajkd.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinhandl ED, Gilbertson DT, Collins AJ. Mortality, Hospitalization, and Technique Failure in Daily Home Hemodialysis and Matched Peritoneal Dialysis Patients: A Matched Cohort Study. Am J Kidney Dis. 2016;67:98–110. doi: 10.1053/j.ajkd.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Molnar MZ, Ravel V, Streja E, et al. Survival of Elderly Adults Undergoing Incident Home Hemodialysis and Kidney Transplantation. J Am Geriatr Soc. 2016;64:2003–10. doi: 10.1111/jgs.14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaber BL, Lee Y, Collins AJ, et al. Effect of daily hemodialysis on depressive symptoms and postdialysis recovery time: interim report from the FREEDOM (Following Rehabilitation, Economics and Everyday-Dialysis Outcome Measurements) Study. Am J Kidney Dis. 2010;56:531–9. doi: 10.1053/j.ajkd.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 13.Twardowski ZJ. Daily dialysis: is this a reasonable option for the new millennium? Nephrol Dial Transplant. 2001;16:1321–4. doi: 10.1093/ndt/16.7.1321. [DOI] [PubMed] [Google Scholar]
- 14.Suri RS, Larive B, Sherer S, et al. Risk of vascular access complications with frequent hemodialysis. J Am Soc Nephrol. 2013;24:498–505. doi: 10.1681/ASN.2012060595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poon CK, Chan CT. Home hemodialysis associated infection-The “Achilles’ Heel” of intensive hemodialysis. Hemodial Int. 2017;21:155–60. doi: 10.1111/hdi.12508. [DOI] [PubMed] [Google Scholar]
- 16.Rivara MB, Soohoo M, Streja E, et al. Association of Vascular Access Type with Mortality, Hospitalization, and Transfer to In-Center Hemodialysis in Patients Undergoing Home Hemodialysis. Clin J Am Soc Nephrol. 2016;11:298–307. doi: 10.2215/CJN.06570615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young BA, Chan C, Blagg C, et al. How to overcome barriers and establish a successful home HD program. Clin J Am Soc Nephrol. 2012;7:2023–32. doi: 10.2215/CJN.07080712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shafi T, Jaar BG. Maintaining Patients on Home Hemodialysis: The Journey Matters as Does the Destination. Clin J Am Soc Nephrol. 2017 doi: 10.2215/CJN.06890617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trinh E, Chan CT. The Burden of Harm–What Is the Ideal Vascular Access for Home Hemodialysis? Clin J Am Soc Nephrol. 2016;11:205–6. doi: 10.2215/CJN.12681115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosner MH, Lew S, Conway P, et al. Perspectives from the Kidney Health Initiative on Advancing Technologies to Facilitate Remote Monitoring of Patient Self-Care in RRT. Clin J Am Soc Nephrol. 2017 doi: 10.2215/CJN.12781216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hothi DK, Stronach L, Sinnott K. Home hemodialysis in children. Hemodial Int. 2016;20:349–57. doi: 10.1111/hdi.12421. [DOI] [PubMed] [Google Scholar]


