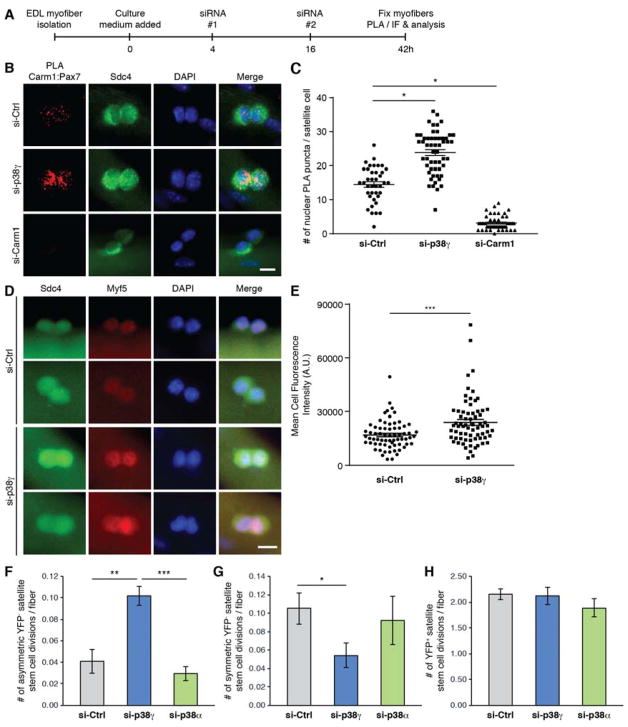
Figure 4. p38γ Negatively Regulates Asymmetric Satellite Stem Cell Division and is Required for Symmetric Self-Renewal.
(A) Schematic of siRNA-treated myofiber culture experiments performed in Figure 4. (EDL, extensor digitorum longus. PLA, proximity ligation assay. IF, immunofluorescence).
(B) Carm1:Pax7 PLA (red) performed on satellite cells cultured for 42h on myofibers that were treated with indicated siRNAs. Satellite cells are marked by expression of Sdc4 (green) and nuclei were counterstained with DAPI (blue). Scale bar represents 10 μm.
(C) Quantification of PLA signal from (B), represented as the mean (n = 39 si-Ctrl, n = 54 si-p38γ, n = 47 siCarm1 cells from 3 mice) ± SEM (*p ≤ 0.05). The PLA was quantified by counting the number of nuclear PLA puncta for each satellite cell.
(D) Immunofluorescence of satellite cells cultured for 42h on myofibers that were treated with either control (si-Ctrl) or p38γ (si-p38γ) siRNA. Cells were immunostained for Sdc4 (green), and Myf5 (red). Nuclei were counterstained with DAPI (blue). Scale bar represents 10 μm.
(E) Quantification of mean fluorescence intensity of Myf5 staining from (D), represented as the mean (n = 67 si-Ctrl, n = 69 si-p38γ cells from 3 mice) ± SEM (***p ≤ 0.001).
(F) Quantification of the number of asymmetric satellite stem cell (Pax7+/YFP−) divisions per myofiber, represented as the mean (n = 9 mice) ± SEM (**p ≤ 0.01. NS, not significant).
(G) Quantification of the number of symmetric satellite stem cell (Pax7+/YFP−) divisions per myofiber, represented as the mean (n = 9 mice) ± SEM (*p ≤ 0.05. NS, not significant).
(H) Quantification of the number of committed satellite progenitor cell (Pax7+/YFP+) divisions per myofiber, represented as the mean (n = 9 mice) ± SEM (NS, not significant).
See also Figure S4.