Abstract
Hydroxyapatite (HA) is an attractive bioceramic for hard tissue repair and regeneration due to its physicochemical similarities to natural apatite. However, its low fracture toughness, poor tensile strength and weak wear resistance become major obstacles for potential clinical applications. One promising method to tackle with these problems is exploiting graphene and its derivatives (graphene oxide and reduced graphene oxide) as nanoscale reinforcement fillers to fabricate graphene-based hydroxyapatite composites in the form of powders, coatings and scaffolds. The last few years witnessed increasing numbers of studies on the preparation, mechanical and biological evaluations of these novel materials. Herein, various preparation techniques, mechanical behaviors and toughen mechanism, the in vitro/in vivo biocompatible analysis, antibacterial properties of the graphene-based HA composites are presented in this review.
Keywords: Graphene, Hydroxyapatite, Composites, Bone tissue, Biomedical devices
Graphical abstract
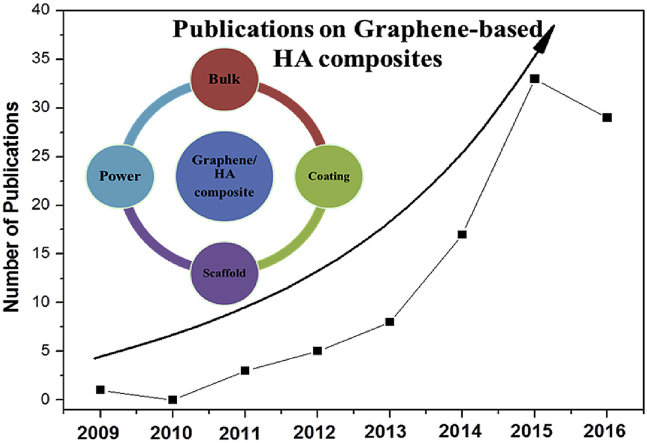
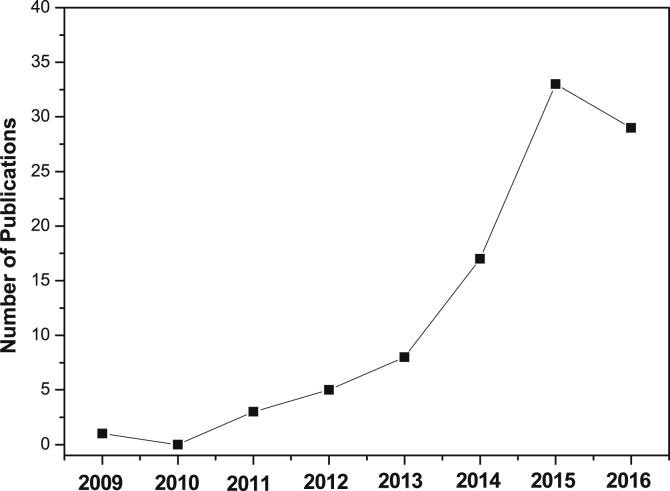
Significant growth in the number of publications graphene-based HA composites (in the form of bulk, powder, coatings and scaffold) from 2013 to 2016 highlights the novelty and importance of this topic in the up-to-date scientific community.
Highlights
-
•
Graphene-based HA composites displayed increased bioactivity and mechanical properties for hard tissue engineering.
-
•
This paper presented a comprehensive review of current achievements and findings in Graphene-based HA composites.
-
•
This paper presented the preparation methods, mechanical properties, and biocompatibility of graphene/HA composites.
1. Introduction
Bone tissues consist of organic and inorganic components, with self-healing ability and great capability to withstand mechanical loading. Fixation of bone fractures and non-unions, correction of spinal deformities and replacement of arthritic joints [1] are major unmet clinical needs. Traditionally, biological approaches for bone repair involve using autografts and allografts of cancellous bone [2]. Nowadays, calcium phosphate ceramics and bioactive glasses are introduced as promising osteoinductive and osteoconductive substitutes for large orthopedic defect remolding or regeneration [3]. In addition, these bioceramics are also utilized as coating on metallic implants to provide long-term performance of the devices and to minimize micromotion between bones and implants during physiologic loading [1,4].
Hydroxyapatite (HA, Ca10(OH)2(PO4)6) possesses chemical and crystallographic similarities to inorganic components of the bone matrix and the teeth [5] with excellent osteoconductivity and osteoinductivity. It has been clinically used as bioactive coatings on dental and orthopedic implants, enabling the adhesion and proliferation of osteoblast cells on the prosthetic surface, and resulting in biological fixation between bone tissues and the implant [6]. However, one primary limitation, when used under major load bearing, is its poor mechanical properties, such as low fracture toughness and tensile strength [5]. To address this problem, specific reinforcing materials, such as carbon nanotubes [7], polyethylene [8], Al2O3 and TiO2 [9], are typically used to prepare HA composites with increased mechanical properties, but these materials result in significantly less bioactivity than that of pure HA.
Graphene is rapidly rising as a promising material for biomedical applications [10,11], featuring two-dimensional nanosheet of hexagonally bonded carbon atoms, with large surface area, high conductivity, strong mechanical properties and good biocompatibility. The graphene-based composites have great advantages when used in bone repair or regeneration, as it can induce osteogenic [[12], [13], [14]] and chondrogenic [15] differentiation of stem cells. Compared with other reinforcement fillers, graphene can greatly increase the mechanical properties of the composite at low content, and its high elasticity and flexibility (adaptability to flat or irregular surfaces) also renders graphene and its derivatives (graphene oxide (GO) and reduced graphene oxide (rGO)) as promising mechanical fillers for biomaterials.
Recently, biomaterial scientists have explored the possibilities of preparing graphene-based HA composite for orthopedic applications with increased bioactivities and mechanical properties. Graphene-based HA composites can be prepared in the form of powders, bulks, coatings and scaffolds. The powders or bulk composites can be used to repair the bone defects or small non-unions. This novel material can also be coated onto orthopedics metallic implant to increase its bone-binding abilities. As for the large defects or bone loss, three dimensional porous graphene-based HA composites can be incorporated into the damaged hard tissues to accelerate their regeneration.
The related research has begun very recently in 2009 [16]. The chronological tendency of the research papers on graphene/HA system is shown in Fig. 1, showing an increasing interest in this area. A significant growth in the number of publications from 2013 to 2016 highlights the novelty and importance of this topic in the up-to-date scientific community. Therefore, in the foreseeable future, more and more related works will be undertaken, and it is the right time to present a comprehensive review of current achievements and findings in this field, which may provide guidance and future directions for further study.
Fig. 1.
The number of publications on Graphene-based HA composites from year 2009–2016 (2009 [16], 2011 [[17], [18], [19]], 2012 [[20], [21], [22], [23], [24]], 2013 [[25], [26], [27], [28], [29], [30], [31], [32]], 2014 [[33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49]], 2015 [[50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82]], 2016 [[83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112]]). This figure does not include the papers published in 2017 [[113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128], [129], [130]].
Herein, we present a comprehensive review on almost all of the available investigations on graphene/HA system. Articles were identified via Web of Science and Google Scholar by searching “graphene” and “hydroxyapatite” which were published up to June 2017. This review paper includes the issues regarding the preparation methods, mechanical properties, in vitro and in vivo biocompatibility of graphene/HA composites, as well as the underlying challenges required to be coped with.
2. Preparation of composites
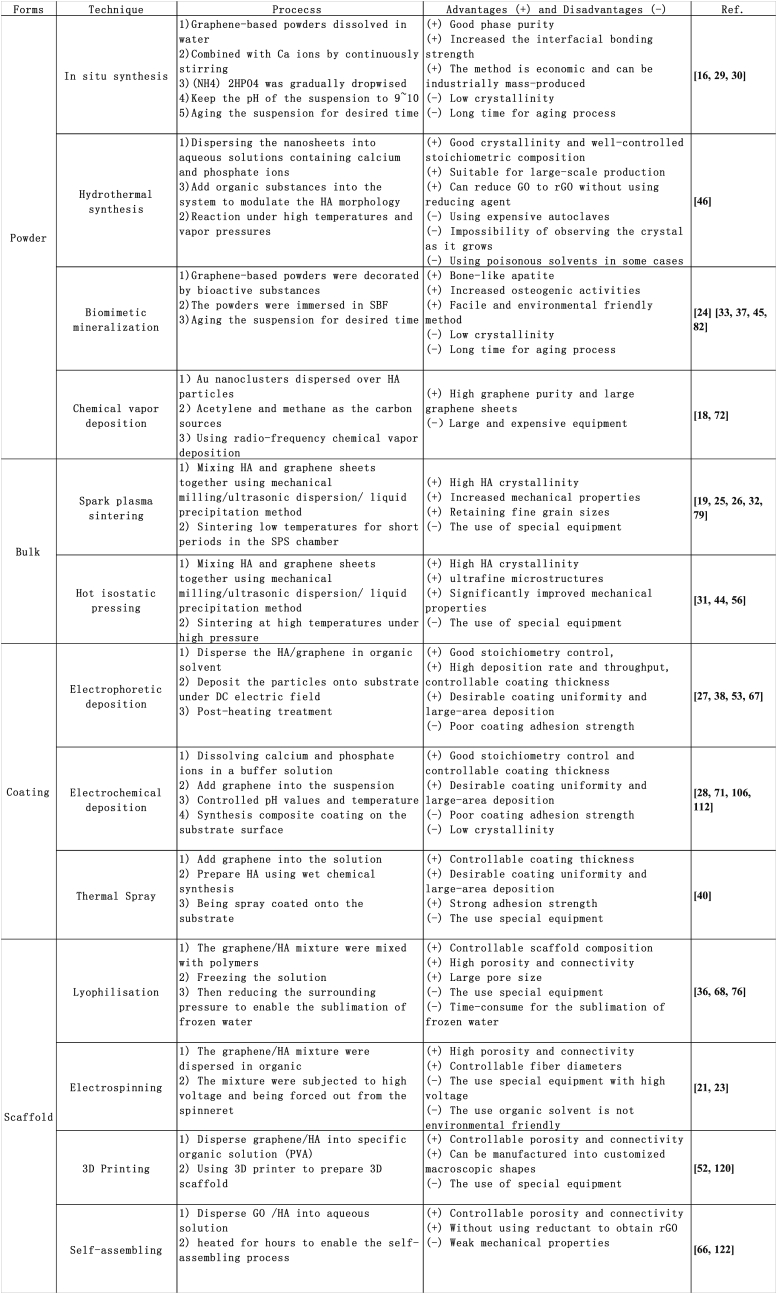
Various preparation techniques could be exploited to make this interesting composites, which is summarized in Fig. 2. In most cases, the composite prepared under high temperature or high pressures have high crystallinity and mechanical properties, such as hydrothermal synthesis, spark plasma sintering and hot isostatic sintering. However, thermal spraying techniques usually lower the crystallinity of the HA coating. HA could be synthesized onto graphene and its derivatives and be directly mixed with these nanofillers by ultrasonic dispersion and ball milling.
Fig. 2.
The summary of different preparation methods of the graphene-based composites.
2.1. Graphene/HA composite powder
2.1.1. In situ synthesis
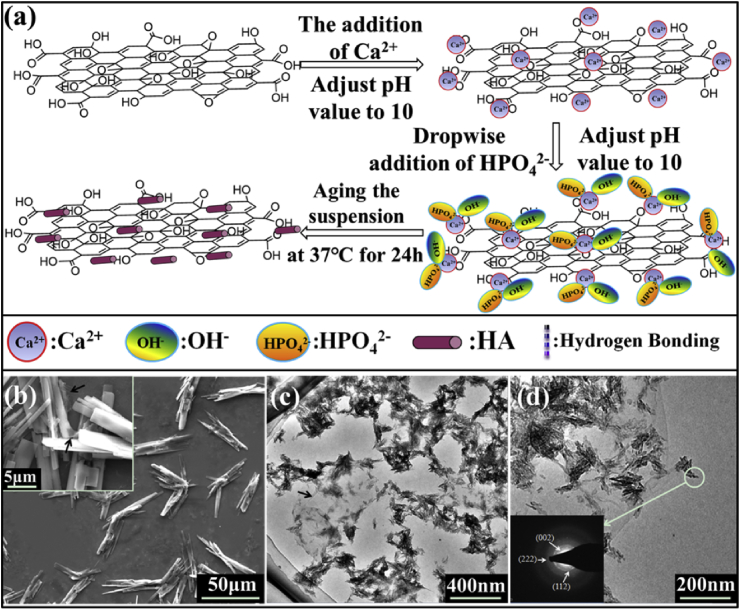
Nano HA particles are successfully fabricated on GO [30], chitosan functionalized GO [30] and rGO [29] surfaces using in situ synthesis methods. Usually, as shown in Fig. 3a, graphene-based powders are first dissolved and exfoliated in DI water by ultrasonic dispersion to obtain a uniform solution; then Ca(NO3)2 is added into the graphene-based solutions by stirring for a desired time; afterwards, the pH of the suspension is adjusted to 9–10 using ammonia water, and (NH4) 2HPO4 was added dropwise into the mixture [30]. The resulting composite solutions are recommended to be aged for days to ensure the fully transformation of apatite into hydroxyapatite with good phase purity and well crystallinity. During the synthesis, the oxygen-containing functional groups on GO surfaces behave as receptor sites for Ca2+ through electrostatic interactions; these anchored cations can in situ react with the phosphate ions to obtain apatite nanoparticles. The underlying reaction mechanism has been proposed and discussed by Li et al. [30]; the distribution and the microstructures of HA on graphene are mainly influenced by (1) the amounts and types of the oxygenous groups on the graphene-based templates and (2) the concentration of the reagents (Ca2+ and HPO42−), solution pH values and so on. Besides, Ca(OH)2 and H3PO4 are also utilized by Gururaj et al. to in situ deposit HA on rGO nanosheets [29].
Fig. 3.
(a) The proposed in situ synthesis mechanism of HA on pristine GO sheets. The SEM and TEM images of GO–HA (b–d) composites, the insets of insets (d) show the selected area electron diffraction (SAED) patterns of the corresponding composites. The black arrows of (b) point to the wrinkles of the GO sheets. These figures were adapted from Ref. [30].
Composite, prepared in this method, is expected to increase the interfacial bonding strength between graphene and HA, facilitating the stress transfer from the matrix to the graphene-based nanofillers. This facile approach is economical and can be industrially mass-produced.
2.1.2. Biomimetic mineralization
Biomimetic mineralization is a facile and environmental friendly method to synthesis bone-like apatite under ambient conditions in aqueous environments. Usually graphene and its derivatives are immersed in a supersaturated or unstable solution with calcium ions and phosphate ions concentrations similar to simulated physiological condition, and apatite will nucleate and precipitate on the surface of those graphene-based materials. During the mineralization process, GO greatly enhance the nucleation and crystallization of HA, resulting in a hybrid homogeneous GO/HA coatings with dense and fine flake-like HA nanocrystalline [54]. Usually, graphene and its derivatives are surface-functionalized by bioactive materials to endow the composite with novel properties and facilitate the biomimetic deposition of HA. The GO can be modified by gelatin to mimic the charged proteins in extracellular matrix for regulating bone formation, and the presence of gelatin improves the attraction of calcium ions and promotes the nucleation of HA [37]. Besides GO can be also biofunctionalized by polydopamine [24], casein phosphopeptide [26], carrageenan [35], chitosan [104,131], fibrinogen [33] or peptide [78] to improve the mineralization process.
2.1.3. Hydrothermal synthesis
Hydrothermal synthesis of graphene/HA composite involves of dispersing graphene or GO into aqueous solutions containing calcium and phosphate ions, and crystallizing HA nanoparticles at high reaction temperatures and vapor pressures. Rod-like HA, with an average length of 55 nm and diameter of 13 nm, has been successfully synthesized on both sides of graphene nanosheets by using the convenient one-pot hydrothermal synthesis strategy [46]. This technique can improve the crystallinity of HA and partially reduce GO to rGO [64]. By using a mixed solvent system of ethylene glycol, N,N-dimethylformamide (DMF) and water, Baradaran et al. [44] synthesized HA nanotubes on rGO without using reducing agents. This technique is suitable for large-scale production of graphene/HA composites with good crystallinity and well-controlled stoichiometric composition, but possesses the disadvantages of using expensive autoclaves, and the inability to observe the crystal as it grows.
2.1.4. Chemical vapor deposition
Chemical vapor deposition is a low cost and scalable technique to prepare graphene films [132]. Novel multicomponent and biocompatible graphene/HA/Au nanocomposites are prepared by using radio-frequency chemical vapor deposition (rf-CVD), with acetylene and methane as the carbon sources [18]. During the deposition process, Au nanoclusters are uniformly dispersed over HA particles with diameters of 2 nm ∼ 7 nm and act as catalyst for graphene synthesis [18]. This research indicates that longer rf-CVD time can result in few-layers graphene with larger dimensions [18,72].
2.2. Graphene/HA composite bulk
2.2.1. Spark plasma sintering
The applications of bulk HA for hard tissue implants are limited by the low mechanical strength of consolidated HA [133]. During the conventional sintering process, HA will dissociate into tricalcium phosphate and tetracalcium phosphate at 1000 °C–1300 °C, and usually the high temperatures and long sintering time can cause grain coarsening behavior, which may deteriorate the mechanical properties of HA [133]. As an alternative method, spark plasma sintering (SPS) is an effective approach for preparing novel nanoceramics at low temperatures for short periods of time, with the advantages of retaining fine grain sizes. Graphene/HA composites are successfully fabricated by SPS. The starting powders used for SPS can be (i) prepared by mixing HA powders/nanoparticles and graphene sheets together using mechanical milling [79] and ultrasonic dispersion [19,25,59] and (ii) synthesized by a liquid precipitation method [32]. Graphene with diameters of several micrometers are uniformly dispersed and embedded within the HA marix and located between the HA crystal grain boundaries without agglomerations [19]. Typical SEM images of the SPS samples were shown in Fig. 4 [25].
Fig. 4.
SEM images of the surfaces of (a) HA, (b) 0.5 wt% graphene/HA composite and (c) 1.0 wt% graphene/HA composite [25].
2.2.2. Hot isostatic pressing
Hot isostatic pressing (HIP) is a traditional technique to densify presintered components, consolidate powders and increase interfacial bonding [134]. It can be exploited to make HA ceramics with ultrafine microstructures and significantly improved mechanical properties [135]. Recently, graphene is introduced into this system as an effective additive for toughening ceramics/composites; novel graphene/biphasic calcium phosphate composite [31], graphene/nickel-doped biphasic calcium phosphate composite [56], and reduced graphene oxide/nanotube hydroxyapatite composite [44] have been successfully fabricated by using HIP technique.
2.3. Graphene/HA composite coating
2.3.1. Electrophoretic deposition
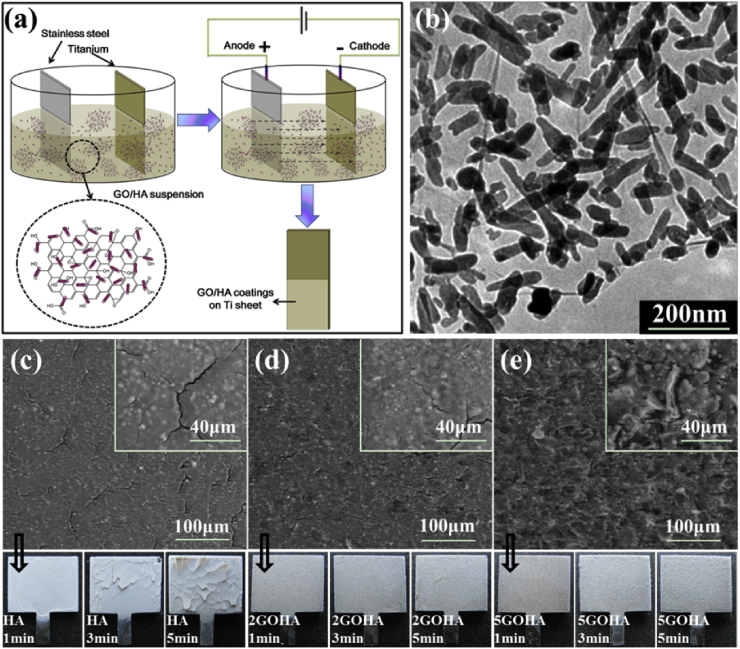
Electrophoretic deposition is a well-developed and aqueous-based colloidal process to deposit charged nano/micro particles onto conductive substrates under DC electric field. It has been widely used to prepare functional bioactive coatings with advanced nanostructures for biomedical applications, such as HA, bioglass and other bioceramic coatings [136]. EPD also enables the fabrication of graphene-based novel composite coatings [137]. In our previous study, GO/HA was successfully prepared on Ti substrate using cathodic electrophoretic deposition [38], as shown in Fig. 5 (a). During the coating process, GO can be considered as amphiphilic macromolecules with an edge-to-center distribution of hydrophilic (oxygenated regions and sheet edges) and hydrophobic (graphenic sp2 hybridized carbon plane) domains [138] to enhance the uniform distribution of HA in the deposition suspensions (Fig. 5 (b)). The 2D novel nanostructure of GO can increase the interlocking of HA nanoparticles and decrease the cracking and delamination the coatings [38]. EPD of HA is usually followed by post-heating treatment to increase the coating/substrate bonding strength and the addition of GO can effectively increase this cohesive strength. The microstructure of the GO/HA composite coating is proposed to be combination of an inner compact layer and outer relatively porous and unsealed layer [38].
Fig. 5.
(a) Schematic illustration of the EPD process (b) The TEM images of the HA nanoparticles with 2 wt% GO sheets (c) The SEM images of composite coatings containing (c) 0 wt%, (d) 2 wt% GO and (e) 5 wt% GO sheets, and corresponding optical photos. These figures were adapted from Ref. [38].
2.3.2. Electrochemical deposition
Electrochemical deposition of HA involves dissolving calcium and phosphate ions in a buffer solution with controlled pH values and temperature under varying electrical current [139]. When the voltage is applied, Ca2+ will migrate onto the cathode surface due to electrostatic attraction and react with the OH− therein produced by the electrolysis of water, resulting in the in situ nucleation and growth of HA on the cathode surface [140]. Zeng et al. [106] fabricate GO/HA coatings on Ti by using this technique; GO was dispersed and mixed with electrolyte for deposition which consist of Ca(NO3)2, NH4H2PO4, NaNO3 (to improve the ionic strength of the solution) and H2O2 (to restrict the formation of hydrogen gas at cathode). The resulting pure HA coating exhibits a rough morphology with shell-like flakes and the GO/HA composite coating shows uniform and porous topography. The increase of GO contents in the electrolyte can improve the HA crystallinity and bonding strength of the coatings.
2.3.3. Thermal spray
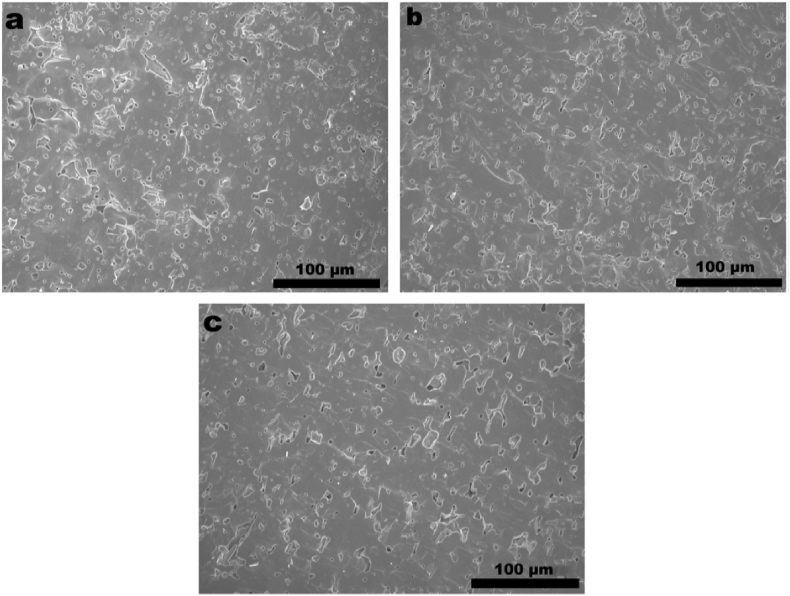
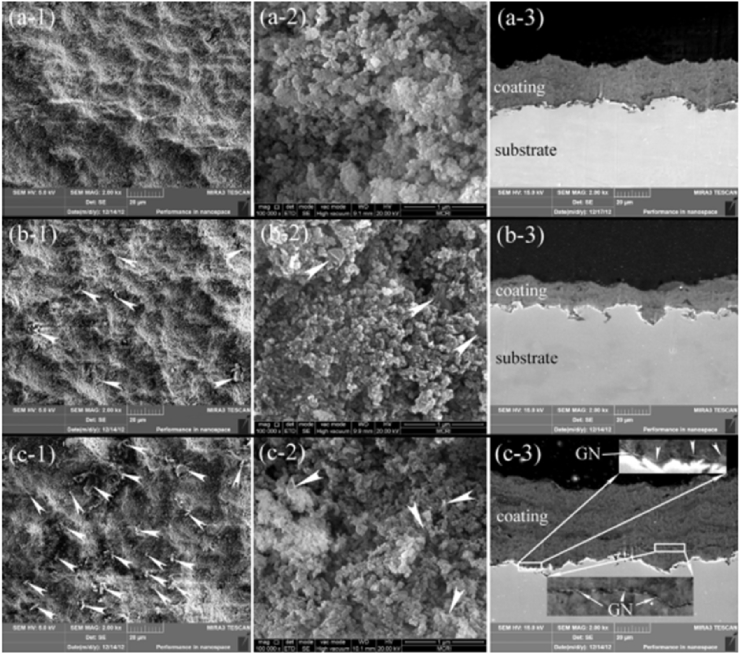
Thermal sprayed HA and HA-based composite coatings have been successfully used on commercially available Ti-based orthopedic implants, having the advantage of high deposition rate, good bonding strength and variable coating thickness [141]. This process involves heating the HA powders to melting state at high temperature, which may cause the decomposition of HA and exhibit detrimental effects on the coating biocompatibilities. Therefore, Liu et al. takes vacuum cold spraying as an alternative to prepare graphene/HA nanostructured coatings at room temperature [40]. The graphene/HA composite powder is prepared by wet chemical approach, and the sprayed coatings have tailorable thickness and display competitive adhesive strength and fracture toughness, with graphene evenly embedded in HA matrix [40]. The FESEM images of the coating were shown in Fig. 6 [40].
Fig. 6.
FESEM views of the as-deposited nanostructured coatings, (a) the pure HA coating, (b) the HA-0.1 wt% graphene coating, and (c) the HA-1.0 wt% graphene coating. −1: surface view, −2: magnified surface view, and −3: cross-sectional view. The white arrow head points to graphene located on the surfaces of the coatings, and magnified views of typical areas from the cross-section of the HA-graphene coating showing clearly the presence of GN in the coating and at the coating/substrate interface (c-3). Graphene -induced layered structure is clearly seen for the HA-graphene coatings.
2.4. Graphene/HA composite scaffolds
Pure graphene 3D porous structures can be achieved by chemical vapor deposition method with Ni foam as template [142] and hydrothermal approach [143] for tissue engineering applications. Moreover, graphene can be utilized as reinforcements for porous nanocomposites and this promising nanocomposite scaffolds can be produced by using lyophilisation or electrospinning.
2.4.1. Lyophilisation
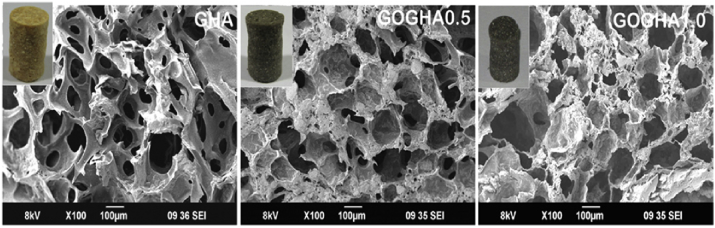
Lyophilisation is a frequently used technique to prepare porous scaffolds by freezing the solution and then reducing the surrounding pressure to enable the sublimation of frozen water. A scaffold with desirable mechanical and biological properties is obtained by lyophilizing the GO, HA and sodium alginate mixtures [76]. This novel nanocomposite scaffolds possess a porosity over 85% and average pore size larger than 150 μm; compared with HA 20 wt%- sodium alginate hybrid scaffolds, the addition of 1 wt% GO can improve their compressive strength and modulus by 23.2% and 28.3% respectively [76]. Nair et al. [68] incorporated the GO nanoflakes into gelatin-HA matrix by using freeze drying method and its morphology was shown in Fig. 7.
Fig. 7.
Scanning electron micrographs of the composite scaffolds. The inset demonstrates the photograph of the scaffolds. Note: GHA: gelatin-HA scaffold, GOGHA 0.5: gelatin-HA scaffold with 0.5 wt% GO, GOGHA1.0: gelatin-HA scaffold with1 wt% GO [68].
2.4.2. Electrospinning
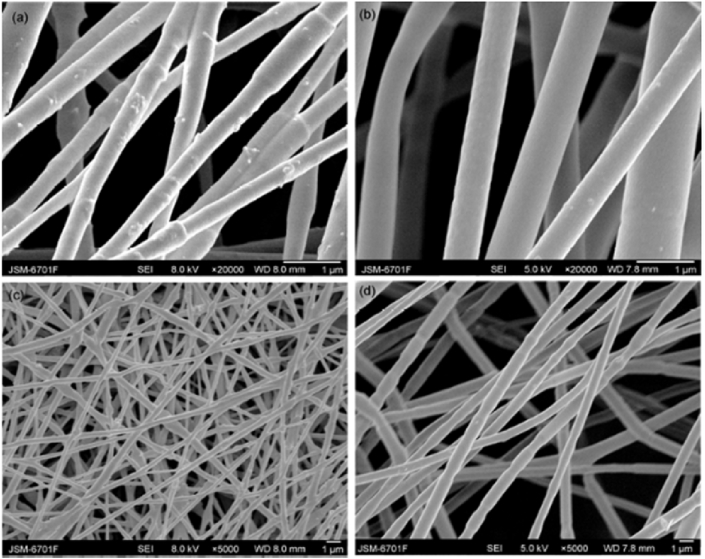
Electrospinning employs an electrical field produced under high voltage to force out the polymeric liquid from the spinneret, resulting in a polymeric fibrous and porous scaffolds on the collectors [144]. Liang et al. prepared a composite nanofiber scaffold consisting of gelatin/chitosan/HA/GO by electrospinning [62] and the effects of the solution composition on fiber morphology were investigated; the addition of GO can obtain nanofibers with uniform and smooth microstructures and endows the fibrous scaffold with good antibacterial effect against both Staphylococcus aureus and Escherichia coli. Ma et al. [23] prepared a porous polylactic acid (PLA)/HA/GO scaffold using electrospinning method and SEM images of the composite were shown in Fig. 8.
Fig. 8.
SEM images of PLA/HA/GO nanofibers with high magnification (a) and low magnification (c), electrospun PLA with high magnification (b) and low magnification (d) [23].
2.4.3. 3D printing
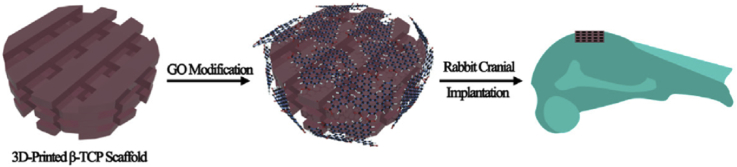
Three dimensional printing is a superior additive manufacturing technique to print scaffold with customized shape, controlled chemistry and porosities and shows great potential for its application in bone tissue engineering [145]. Although bone has self-healing abilities, the large bone loss or damage cannot be healed completely and spontaneously. A scaffold or matrix materials should be incorporated to assist this healing process. Wu et al. [69] prepared GO surface modified β-tricalcium phosphate (β-TCP) scaffolds by first using 3D printing method and then soaking the β-TCP scaffold into GO/water suspension as shown in Fig. 9.
Fig. 9.
Scheme illustration for GO modification of b-TCP bioceramics stimulates the in vivo osteogenesis [69].
2.4.4. Self-assembling
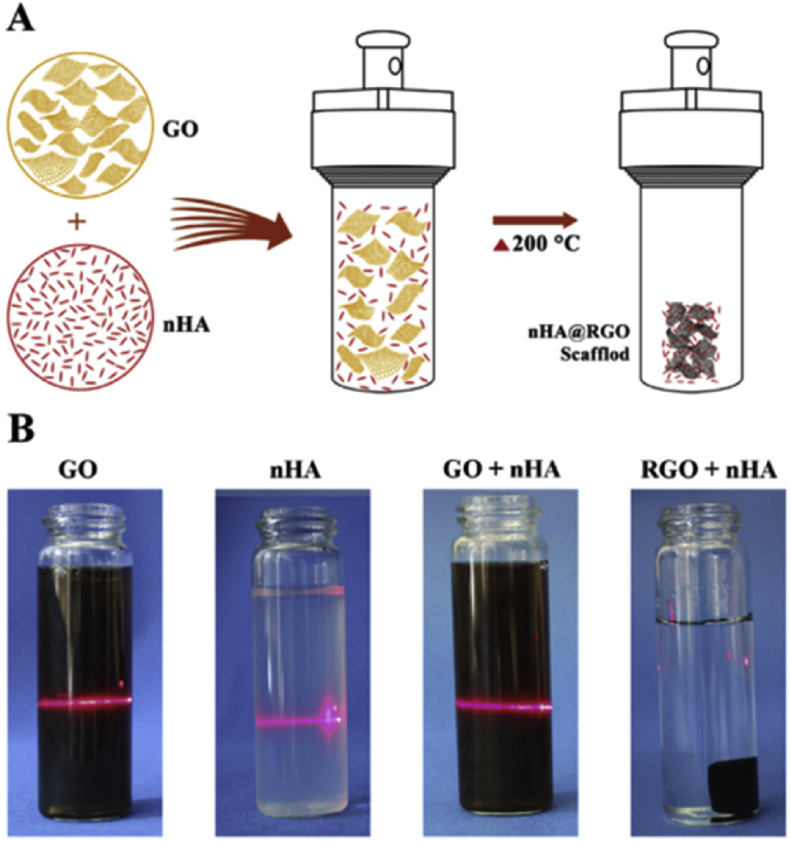
Self-assembly of GO provides a facile and efficient method to produce graphene-based macrostructures. As shown in Fig. 10, GO and HA nanoparticles (nHA) were ultrasonically mixed in ice bath resulting in a homogeneous suspension, and then the mixture were heated at 200 °C for 3 h to induce self-assembly [122]. This technique reduced GO to rGO without using reductant and organic solvent, which could minimize cytotoxicity of the composite.
Fig. 10.
(A) Schematic showing that the self-assembly of RGO and nHA to form a porous RGO scaffold for cranial bone defect reconstruction; (B) Tyndall effect of before and after reaction [122].
3. Mechanical properties of composites
3.1. Mechanical properties of graphene and hydroxyapatite
As a biologically active calcium phosphate ceramic, its poor mechanical properties, such as low fracture toughness and tensile strength, limit its applications in major load-bearing scenario. Therefore, the main goal to incorporate graphene and its derivatives into HA is to improve the overall mechanical properties of the composite, in order to better fulfill its biological functions.
The atomically perfect monolayer graphene displayed a Young's modulus of 1.0 TPa and a fracture strength of 130 GPa, which was predicted to be stronger than any other materials [146]. The mechanical properties of graphene are influenced by the structural defects (monatomic vacancies and Stone-Wales dislocations) [147] and doping or functionalization defects [148]. As the oxygenated derivatives of graphene, graphene oxide contains reactive oxygenic groups, rendering it higher chemical activities for surface modification [149] but with lower effective Young's modulus (207.6 ± 23.4 GPa) [150]. GO could be reduced to reduced graphene oxide (rGO) with higher Young's modulus of 0.25 TPa [151]. Compared with pristine graphene, its oxygenated derivatives (GO and rGO) displayed higher stabilization behavior in aqueous media and could be attractive and promising nanoscale reinforcement fillers in biocomposites [38]. These nanofillers could enhance the interfacial bonding within the components, and facilitate stress transfer in the composites [152].
3.2. Mechanical properties of composites
Composites are expected to exhibit improved properties than their individual components. The addition of GO could efficiently increase the adhesion strength of the HA coatings. The binding strength between the GO/HA coating and Ti substrate were evaluated according to ASTM F1044-99 in our previous research [30], and compared with pure HA coating, the adhesion strength of 5 wt%GO/HA coating increased from 1.55 ± 0.39 MPa to 3.3 ± 0.25 MPa.
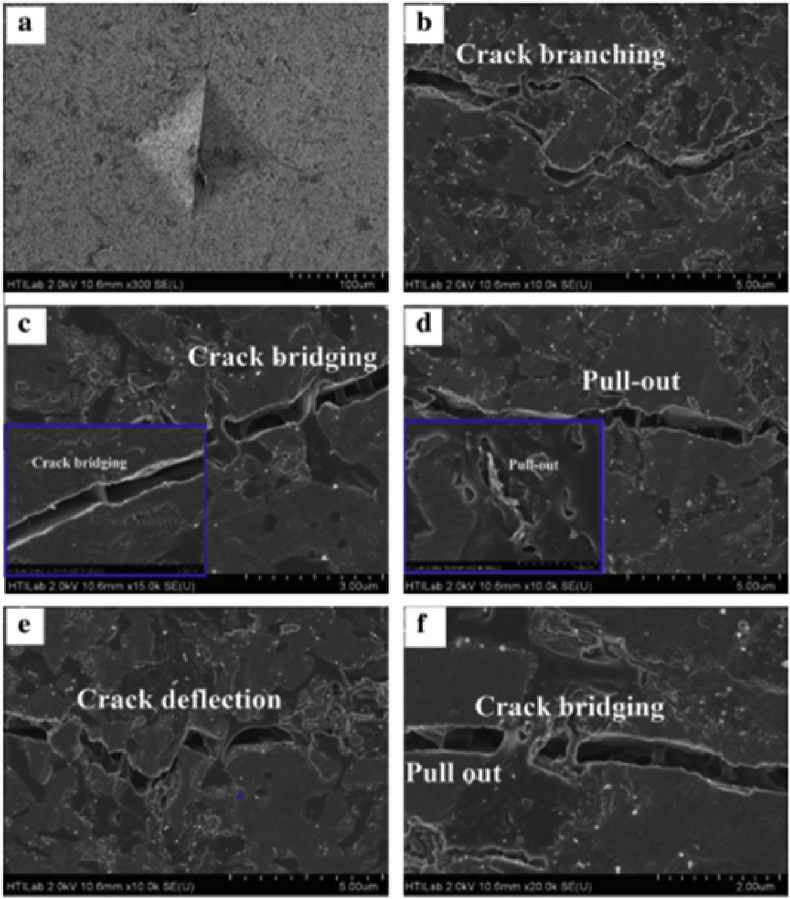
The rGO/HA composites were prepared by hydrothermal approach and consolidated by hot isostatic pressing technique; and then their mechanical properties were assessed using the indentation method as shown in Fig. 11 [28]. According to the investigation of S. Baradaran et al., even low addition of rGO has a great effect on the bulk mechanical properties, and the composite containing 1.5 wt% rGO displayed a maximum fracture toughness and elastic modules, around 86% and 40% higher than that of the pure HA [28].
Fig. 11.
Characteristic toughening mechanisms at a striation line in the composites with 1.5 wt% rGO: Vicker's indentation craters (a) and radial cracks: crack branching (b), crack bridging (c and f), pull out (d and f), crack deflection (e) [44].
3.3. Toughening mechanism
The mechanical efficiency of the reinforcement fillers in the composites were mainly determined by several factors:(1) the intrinsic mechanical properties of the fillers, (2) the inherent mechanical behavior of the matrix, (3) the fillers volume fraction, (4) the preparation method of the composites, (5) the interfacial bonding strength between the filler and matrix, and (6) the distribution level of the fillers within the matrix [44,153].
The toughening mechanism for the graphene-based HA composites, according to previous investigations [25,31,32,44], are crack branching, crack bridging, pull out, crack deflection and other reinforcing mechanisms. To be specific:
-
(1)
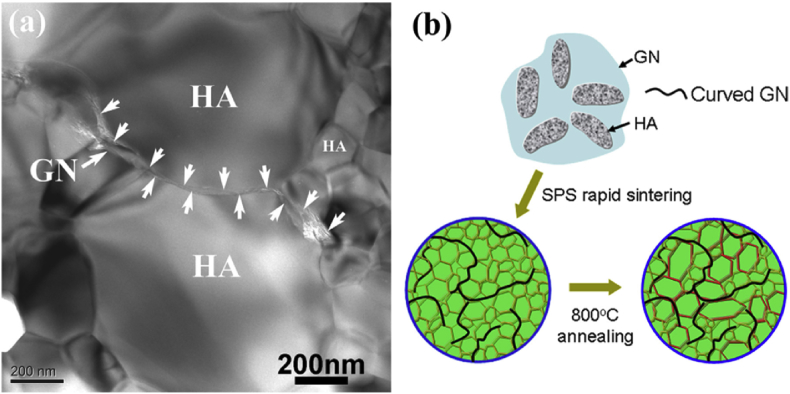
The addition of the 2D fillers can result in the formation of HA with smaller sizes [32,154];and the GO/rGO may functionalized as nucleus for the crystallization of HA crystals [30], which in turn may restrain the growth of HA nanoparticles. It is reported that the fine grain strengthening mechanism could be a possible cause for the improved mechanical properties of the HA composites [32]. This inhibitory effect on grain growth was well studied by Liu et al. [90] and illustrated in Fig. 12.
-
(2)
Moreover, the calculated pullout energy of graphene from HA is around 3–40 J m−2, which is much higher than the fracture energy (1 J m−2) of bulk HA [32]. Therefore, the indentation-induced cracks, as shown in Fig. 11, preferably propagate within the HA matrix, instead of along the HA-graphene interface. The stress transfer characteristics in graphene-based nanocomposites were evaluated from a computational model using a multi-scale finite element approach [102].
-
(3)
Graphene-based nanosheets have high specific surface area enabling increased contact area with the matrix [25]. And due to its high Young's modulus and flexibility, graphene may be located around the grain boundaries and aligned with the grain shape [31], which is expected to enhance the mechanical interlocking and adhesion strength within the matrix [30]. The graphene-based 2D reinforcement fillers can inhibit the propagation of the crack. Liu et al. [40] prepared graphene/HA coatings by vacuum cold spraying and the authors incubated the samples in culture media for one month without cells, and the coating adhesion values showed no remarkable changes.
Fig. 12.
Restrained HA grain growth by graphene and abnormal growth of the grain along the direction parallel to graphene, (a) TEM image of the HA-graphene composites showing that graphene is predominantly located at the HA grain boundaries, forming a serial wall zones isolating individual HA grains, and abnormal HA grain growth is seen along the direction parallel to graphene, and (b) schematic depiction of the composites illustrating evolvement of the HA grains during the SPS processing and following heat treatment.
4. Biocompatibility of the composites
4.1. In vitro biocompatibility
From a mechanical point of view, this graphene-based HA nano-structural composites have proven to be promising candidates for hard tissue repairing or regeneration. Furthermore, these materials should be biocompatible with high stability in biological environment. Their toxicity was well analyzed and studied.
4.1.1. Cytocompatibility
Usually the graphene-based HA composites were co-cultured with (1) osteoblast-related cells: mouse pre-osteoblast cell line (MC3T3-E1) [[17], [18], [19],23,25,35,37,46,71,74], human fetal osteoblastic cell line (hFOB 1.19 cells) [39,40,44,56], human osteosarcoma cell line(MG63 cells) [30,34,38,155]; (2) fibroblast-related cells: mouse embryonic fibroblast cell line (NIH 3T3 cells) [34], mouse fibroblast cell line (L929 cells) [24,30,33,38], (3) stem cells: mesenchymal stem cells (MSC) [28] and (4) other cells: PBMC (human peripheral blood mononuclear cells) [53,67].
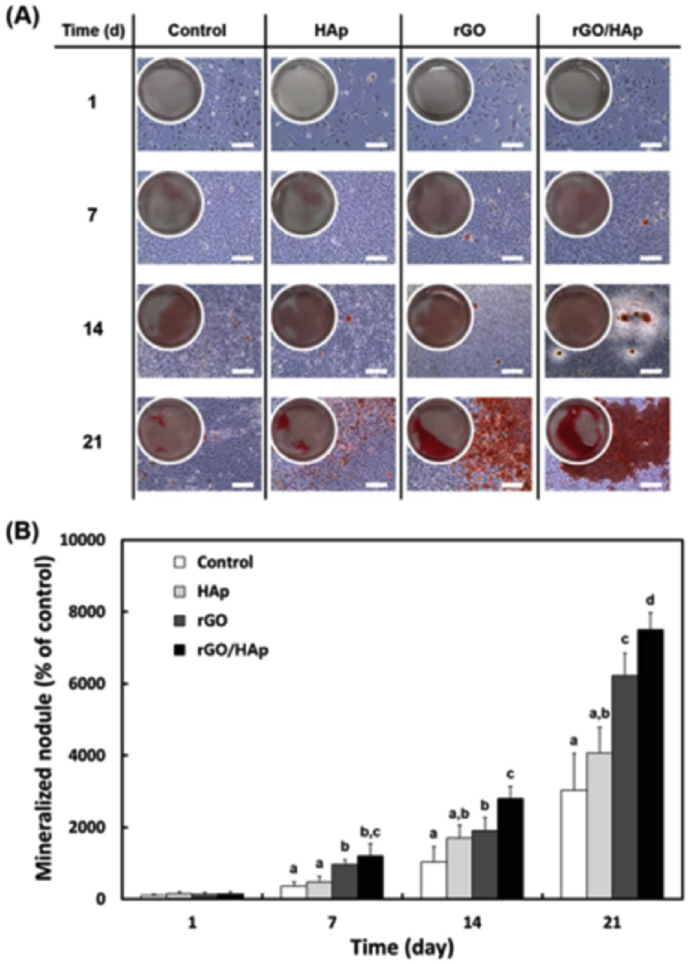
Fan et al. [46] seeded MC3T3-E1 cells onto graphene/HA composites with various HA contents and the cells displayed flatter morphology than on the GO and HA coating, and the composite containing 40 wt% showed higher bone cellular activities. In order to further increase the biocompatibility of the graphene/HA composites, bioactive polymers were added into the system, such as carrageenan [35], gelatin [37] and polydopamine [74]. Combined with the graphene-based fillers, these polymers could facilitate the HA mineralization process which promoted the osteogenic differentiation of MC3T3-E1 cells. Lee et al. [63] cultured the MC3T3-E1 cells (2 × 105 cells/mL) with colloidal dispersion of HA particles, rGO nanosheets and rGO/HA nanocomposites (10 μg/mL) for 1–21days and the cells were analyzed by Alizarin red staining (ARS). The results were shown in Fig. 13.
Fig. 13.
The ARS stain and its corresponding extract in MC3T3-E1 cells incubated with a colloidal dispersion of HAp particles, rGO nanosheets or rGO/HAp nanocomposites in basal media. (A) Increased calcium deposits by rGO/HAp nanocomposites were not related to the cell number (scale bars = 200 μ m). There was a notable formation of calcium deposits by rGO/HAp nanocomposites from 14 d. (B) The dissolved ARS extracted from the staining plates confirmed that the rGO/HAp nanocomposites significantly (p < .05) increased extracellular calcium deposition in the cells [63].
Oyefusi [39] investigated the hFOB 1.19 cell proliferation and differentiation by using total protein assays and Western blot analysis of osteocalcin expression with promising results. Liu et al. [40] cultured the osteoblast cells on the composite coatings showing higher proliferation rate and better stretching behavior on the HA-based coatings than on bare Ti. The random curly incorporated graphene sheets possessed more active sites for cell binding and could adsorb the key serum proteins (fibronectin) to further enhance the cell attachment [40].
The graphene/HA composites also showed good cytocompatibility to MG63 cells. The HA coated GO exhibited comparable biocompatibility to HA minerals [155]. However, high GO contents in the composites might inhibit cell viabilities [38]. Compared with the GO/HA composites, the addition of chitosan could increase its bone-forming abilities [30]. Ramani et al. [34] prepared bacterial cellulose/GO/HA composites with high osteoinductive and the MG63 cells cultured with 50 μg/mL composites had higher ALP activities.
Liu et al. [24] evaluated the cytotoxicity of rGO/HA on L929 cells using MTT assay and the cell viability was more than 95% in comparison with the control; moreover, the concentration of this composite in the culture media (0, 1, 5, 10, and 20 μg/mL) had no significant influences on the mitochondrial activities within the L929 cells. Wang et al. [33] prepared a GO/fibrinogen nanofiber scaffold by using layer-by-layer method and then incubated it in SBF for biomineralization resulting in 3D GO/HA scaffolds, which had no obvious inhibitory effects on the in vitro cell proliferation of L929 cells. In our previous study [30,38], the in vitro cytotoxicity of the GO/HA and chitosan modified GO/HA did not exhibit obvious concentration-dependent characteristics but showed a clear positive time dependence [30]; and the cells displayed a healthy round shape with extended pseudopodium [38].
Zanin et al. [28] prepared globular nano-HA onto rGO by electrodeposition and evaluated the composite's biocompatibility by culturing with MSC. The cells adhered well on the composites with a flat roughly circular morphology, presenting active formation of membrane projections. PBMC consists of lymphocytes and monocytes, as the main representatives of human immune cells [53]. Compared with the control group, the cells viability rate on the composites showed mild decrease and the composite could be considered non-toxic.
4.1.2. Hemocompatibility
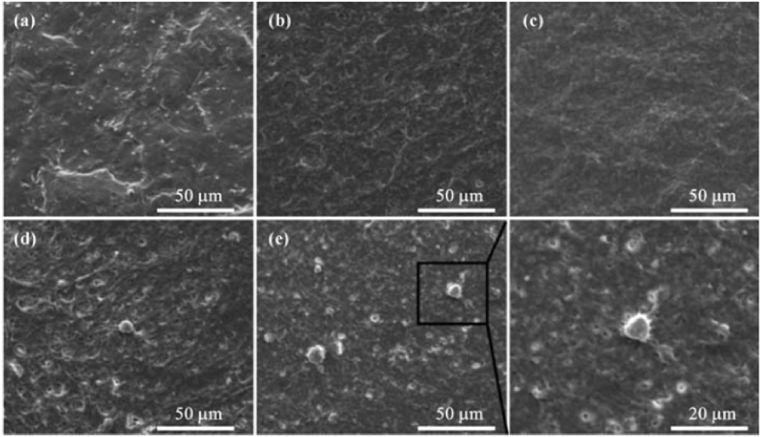
Nair et al. [68] incorporated the GO nanoflakes into gelatin-HA matrix by using a freeze drying method. None of the prepared scaffolds showed hemolysis with comparable activity to the saline control groups [68]. In our previous study [88], silk fibroin/GO/HA coatings were electrophoretic-deposited on Ti substrates and the hemocompatibility of samples were evaluated according to ASTM F756-08 standard [156]. All the samples exhibited good hemocompatibility with hemolysis rate lower than 5%, and platelet adhesion tests showed that only few platelets were observed on the composite coatings at the inactivated stage with round shape (Fig. 14). The GO and graphene nanosheets showed a dose-dependent hemolytic activity on red blood cells [157] and covering GO sheets with HA could alleviate their hemolytic activity.
Fig. 14.
SEM images of the adhered platelets on pristine (a) Ti, (b) SF, (c) SF/GO, (d) SF/HA and (e) SF/GO/HA coatings [88].
4.1.3. Mineralization abilities
Simulated body fluid (SBF) contains ion concentrations almost equal to those of human blood plasma. Usually the bone-forming or bone-bonding abilities of a material could be evaluated by immersing it into SBF to examine apatite formation abilities on its surface, which is a useful method to predict the in vivo osteogenic activity of a material [158]. However, some researchers suggested that this method needs further elaboration [159].
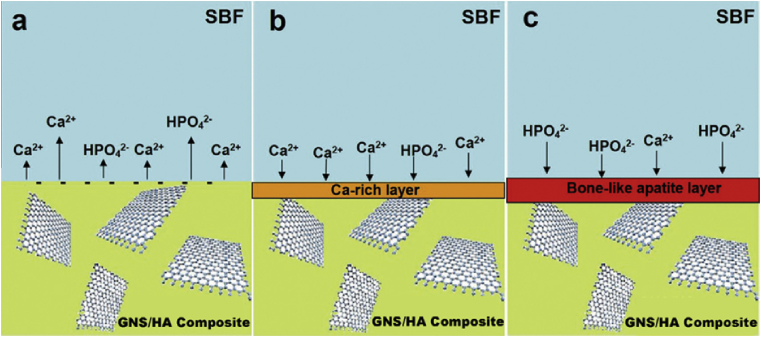
The addition of graphene-based nanofillers into HA matrix could accelerate the formation of apatite on its surface [25,42,46,53,78]. Zhang et al. [25] prepared graphene/HA composites using spark plasma sintering technique. The samples were immersed in SBF for 7 days, and, compared with the HA, the bone-like apatite layer formed on 1.0 wt% graphene/HA was much thicker than that on the pure HA substrate, probably suggesting a higher osteogenic activity of the graphene-reinforced HA [25]. The proposed mineralization process were depicted in Fig. 15. Compared with pure HA, the graphene/HA composites exhibited lower stability. The incorporation of graphene into HA could result in smaller grain size and more specific area, leading to fast dissolution of calcium and higher negative surface charge on the composites (Fig. 15 a). Therefore, more calcium could be attracted on its surface (Fig. 15 b) and the Ca-rich layer could generate thicker bone-like layer (Fig. 15 c) [25,53]. In order to further increase the bone-forming ability of the composites, the nanosheets could be coated by bioactive polymers such as polyethylene glycol [42] and self-assembled peptide nanofibers [78].
Fig. 15.
Schematic illustration of the mineralization process for a GNS/HA composite immersed in SBF. (a) Dissolution controlled stage, in which calcium ions dissolution is predominant, leading to negatively charged surface. (b) Precipitation controlled stage, in which negatively charged surface together with more nucleation sites attract calcium ions from SBF to form the Ca-rich layer. (c) Formation of bone-like apatite, in which Ca-rich layer attracts phosphate ions and form bone-like apatite [25].
4.2. In vivo biocompatibility
Bioactivity of the graphene-based HA composites in the context of osteogenesis by using in vitro cell models have been extensively investigated. However, there were few in vivo animal studies using these novel material.
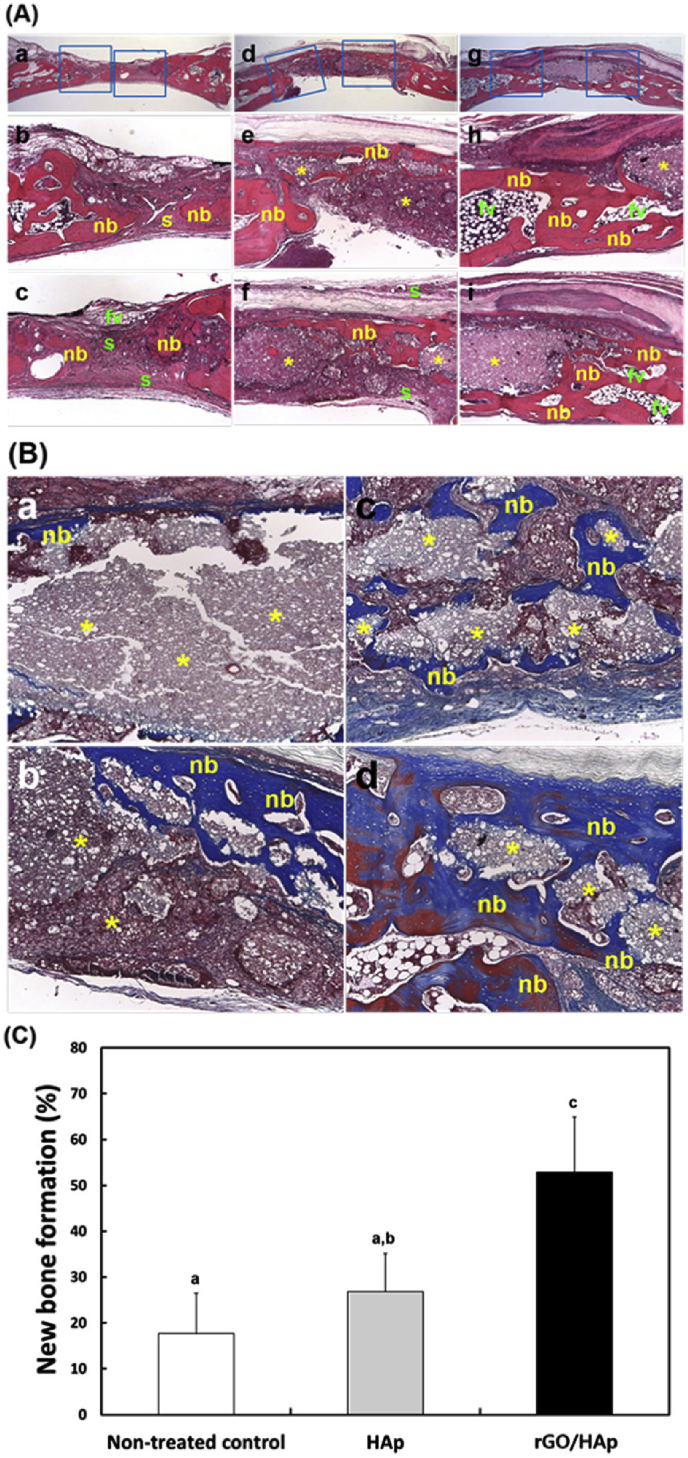
Lee et al. [63] prepared the HA grafts and the rGO/HA grafts. The grafts were filled into bone defects (6 mm in diameter and 2.5 mm in depth) that were trephined in the parietal bone of 12–13 week-old male New Zealand rabbits [63]. Four weeks after the surgery, the non-treated control defects were filled with thin and loose connective tissues without many new bones (Fig. 16A(a-c)), and dense connective tissue and small particles were observed in the one with HA grafts (Fig. 16 A(d-f)). For the groups implanted by rGO/HA grafts (Fig. 16A(g-i)), several newly formed bone were detected, indicating the accelerated bone remodeling process. From the Masson's trichrome staining analysis, the amount of the newly formed bone in the rGO/HA treated defects (Fig. 16 B(c,d)) were substantially higher than that in the HA groups (Fig. 16 B(a,b)). The histometric evaluations (Fig. 16 C) showed that the rGO/HA grafts displayed significantly greater new bone density than the control/HA groups.
Fig. 16.
Histological observations. (A) Images from HE staining: non-treated control (a–c), the defects treated with the HAp grafts (d–f), the defect treated with rGO/HAp grafts (g–i) Original magnifications: × 12.5 in (a,d,g) and × 50 in the others. (B) Images from MT staining. (a,b) HAp grafts and (c,d) rGO/HAp grafts. Original magnifications: × 100. Symbols: nb, new bone; s, soft tissue; fv, fibrovascular tissue; *, graft materials. (C) New bone formation (%) [63].
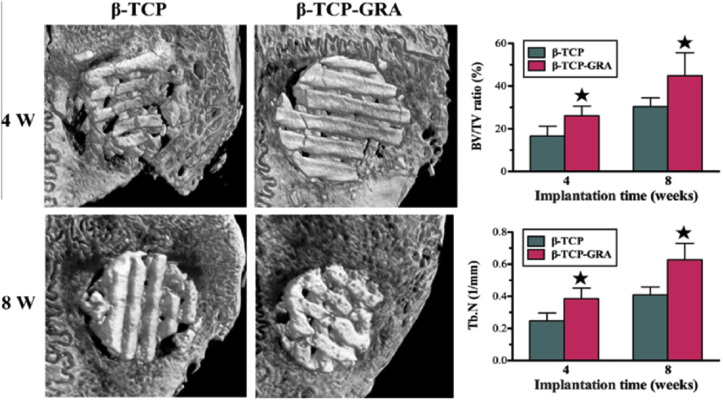
Wu et al. [69] prepared GO modified β-tricalcium phosphate (β-TCP) scaffolds by first using 3D printing and then soaking the β-TCP into GO/water suspension. The in vivo bone formation of the scaffold was evaluated by implanting it into critical-sized calvarial defects in New Zealand white male rabbits. Compared with β-TCP scaffolds, the GO modified scaffolds had greater bone formation abilities in the defects both at 4 and 8 weeks post implantation. And the new bone was visible in the periphery and center of the defect in the GO-β-TCP group, which was both quantitatively and qualitatively better than that in the control group as it is shown in Fig. 17.
Fig. 17.
Micro-CT analysis of in vivo bone formation ability for β-TCP and β-TCP-GRA scaffolds after being implanted in the cranial bone defects of rabbits for 4 and 8 weeks.*Significant difference between the b-TCP and b-TCP-GRA groups (p < .05). Note: β-TCP-GRA represents GO-modified β-tricalcium phosphate andβ-TCP represents β-tricalcium phosphate [69].
Both the in vitro and in vivo biocompatible analysis suggested that the graphene-based HA composites showed great potential applications for bone reconstruction, due to its beneficial effects on the adhesion, proliferation and differentiation of osteoblast-related cells, fibroblast-related cells or stem cells. Graphene and its derivatives displayed strong non-covalent binding abilities on osteogenic inducers (dexamethasone and β-glycerolphosphate) [160] which accelerated the osteogenic differentiation of stem cells.
4.3. Antibacterial property
The infections of the implantation site after surgery [67] and biomedical devices associated infections (especially with the formation of biofilm) [100,161] are the major cause for delayed healing, implant failure and repeated surgeries [161]. Therefore, it is necessary to develop novel composite materials with combined bioactivity and antibacterial properties.
In addition to the bone-forming ability, HA shows favorable affinity for bacterial adhesion [100]. When HA was exploited as coating materials on Ti implants, the increased HA contents therein resulted in enhanced attachment of the bacteria, which could deteriorate its osteointegration property and further weaken the biological fixation ability of HA-coated implants in hard tissues [162]. Accordingly, it is imperative to endow HA with antibacterial or bacteriostatic effects by preparing HA composites. Graphene-based nanomaterials exhibit excellent cytotoxicity to bacteria [163]. Liu et al. [164] investigated the antibacterial activity of four types of graphene-based materials (graphite, graphite oxide, GO and rGO) toward an Escherichia coli and the GO dispersion displayed the highest antibacterial activity. The membrane stress induced by the nanosheets warping [164], the cell membrane damage [165] caused by direct contact with sharp edges of the nanosheets and the oxidative stress generated by the reactive oxygen species (ROS) production [164] were the major mechanisms in the bacterial inactivation.
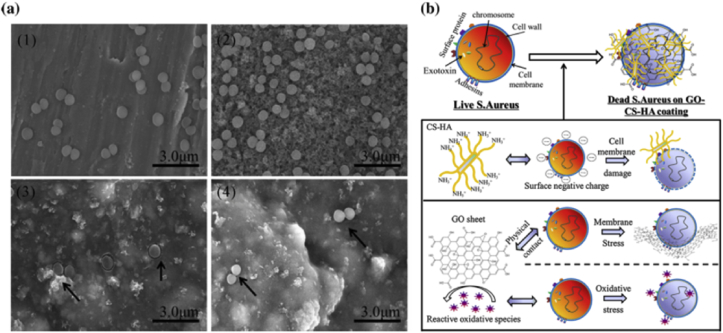
Janković et al. [53] investigated the antibacterial efficiency of the electrophoretic graphene/HA coatings against the Gram-positive pathogenic bacteria strain Staphylococcus aureus and the Gram-negative bacteria strain Escherichia coli. However no antibacterial effects were observed in that study [53] which might be ascribed to the low contents of the incorporated graphene. As an alternative method, Ag was introduced into the coatings, and the graphene/Ag/HA coatings showed strong antibacterial activity only after 3 h co-culturing with the S. aureus and E. coli, inhibiting harmful biofilm formation [67]. In our previous research GO reinforced chitosan/HA coatings were deposited onto Ti and the antibacterial adhesion assay indicated that the amount of the adherent bacterial cells decreased greatly on the composite coatings compared with pure HA coatings [100]. As shown in Fig. 18 (a), compare with Ti and HA coating, the number of the adherent bacterial cells is significantly decreased on the chitosan/HA and GO/chitosan/HA coatings, with the potential antibacterial mechanism displayed in Fig. 18 (b) [100].
Fig. 18.
SEM images (a) of the S. aureus after incubation 12 h with the pristine Ti (1) and different coating interfaces: HA (2), chitosan/HA (3), GO/chitosan/HA (4). The black arrows in (3), (4) point to the adherent S. aureus. Scale bar 3 μm. Schematic illustration (b) of the speculated antibacterial adhesion mechanism of the GO/chitosan/HA nanocomposites.
The novel graphene-based HA composites were competitive candidates for hard tissue repairing or regeneration with increased osteogenic activity, well hemocompatibility, and promising antibacterial properties. However, in some cases the resulting composites exhibited less bioactivity [24,38,53]. This discrepancy may originated from the preparation techniques of graphene, the size and distribution of this 2D nanoscale filler. In the scenario of graphene oxide, the types of oxygen-containing functional groups and the oxygen content could also affect the bioactivity of the composites. Therefore, more researches should be conducted to shed light on this issues.
5. Conclusions
This review of literature presented various preparation techniques, mechanical behaviors and toughen mechanism, in vitro/in vivo biocompatible analysis, antibacterial properties of the graphene-based HA composites for orthopedic applications. By combining the superior mechanical properties of graphene (and its derivatives) and the high bioactivities of HA, the graphene-based HA composites show great potentials in hard tissue repair and regeneration. The major problem in production of this novel composite is the difficulty in homogeneous distribution of the 2D reinforcement fillers within the matrix. Evaluation of the composites mechanical properties has been the focus of the research. Biocompatibility of graphene-based HA composites has been studied mostly in terms of in vitro cytocompatibility, and further in vivo analysis should be conducted before being considered for clinical applications.
Acknowledgement
This work was supported by National Natural Science Foundation of China (No. 31370954, 31670974) and Beijing Natural Science Foundation (2164073).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Goodman S.B., Yao Z., Keeney M., Yang F. The future of biologic coatings for orthopaedic implants. Biomaterials. 2013;34:3174. doi: 10.1016/j.biomaterials.2013.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burg K.J., Porter S., Kellam J.F. Biomaterial developments for bone tissue engineering. Biomaterials. 2000;21:2347. doi: 10.1016/s0142-9612(00)00102-2. [DOI] [PubMed] [Google Scholar]
- 3.El-Ghannam A. Bone reconstruction: from bioceramics to tissue engineering. Expet Rev. Med. Dev. 2005;2:87–101. doi: 10.1586/17434440.2.1.87. [DOI] [PubMed] [Google Scholar]
- 4.Wang G., Zreiqat H. Functional coatings or films for hard-tissue applications. Materials. 2010;3:3994–4050. doi: 10.3390/ma3073994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou H., Lee J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011;7:2769–2781. doi: 10.1016/j.actbio.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Mohseni E., Zalnezhad E., Bushroa A.R. Comparative investigation on the adhesion of hydroxyapatite coating on Ti–6Al–4V implant: a review paper. Int. J. Adhesion Adhes. 2014;48:238–257. [Google Scholar]
- 7.Lahiri D., Singh V., Keshri A.K., Seal S., Agarwal A. Carbon nanotube toughened hydroxyapatite by spark plasma sintering: microstructural evolution and multiscale tribological properties. Carbon. 2010;48:3103–3120. [Google Scholar]
- 8.Fang L., Leng Y., Gao P. Processing and mechanical properties of HA/UHMWPE nanocomposites. Biomaterials. 2006;27:3701–3707. doi: 10.1016/j.biomaterials.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Ramires P., Romito A., Cosentino F., Milella E. The influence of titania/hydroxyapatite composite coatings on in vitro osteoblasts behaviour. Biomaterials. 2001;22:1467–1474. doi: 10.1016/s0142-9612(00)00269-6. [DOI] [PubMed] [Google Scholar]
- 10.Geim A.K., Novoselov K.S. 2009. The Rise of Graphene. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y., Nayak T.R., Hong H., Cai W. Graphene: a versatile nanoplatform for biomedical applications. Nanoscale. 2012;4:3833. doi: 10.1039/c2nr31040f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nayak T.R., Andersen H., Makam V.S., Khaw C., Bae S., Xu X.F., Ee P.L.R., Ahn J.H., Hong B.H., Pastorin G., Ozyilmaz B. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. Acs Nano. 2011;5:4670–4678. doi: 10.1021/nn200500h. [DOI] [PubMed] [Google Scholar]
- 13.Crowder S.W., Prasai D., Rath R., Balikov D.A., Bae H., Bolotin K.I., Sung H.-J. Three-dimensional graphene foams promote osteogenic differentiation of human mesenchymal stem cells. Nanoscale. 2013;5:4171–4176. doi: 10.1039/c3nr00803g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee W.C., Lim C.H.Y.X., Shi H., Tang L.A.L., Wang Y., Lim C.T., Loh K.P. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. Acs Nano. 2011;5:7334–7341. doi: 10.1021/nn202190c. [DOI] [PubMed] [Google Scholar]
- 15.Yoon H.H., Bhang S.H., Kim T., Yu T., Hyeon T., Kim B.S. Dual roles of graphene oxide in chondrogenic differentiation of adult stem cells: cell-adhesion substrate and growth factor-delivery carrier. Adv. Funct. Mater. November 5, 2014;24(41):6455–6464. [Google Scholar]
- 16.Rodríguez-Lorenzo L.M., Benito-Garzón L., Barroso-Bujans F., Fernández M. Synthesis and biocompatibility of hydroxyapatite in a graphite oxide matrix. Key Eng. Mater. 2009;396–398:477–480. [Google Scholar]
- 17.Kim S., Ku S.H., Lim S.Y., Kim J.H., Park C.B. Graphene-biomineral hybrid materials. Adv. Mater. 2011;23:2009–2014. doi: 10.1002/adma.201100010. [DOI] [PubMed] [Google Scholar]
- 18.Biris A.R., Mahmood M., Lazar M.D., Dervishi E., Watanabe F., Mustafa T., Baciut G., Baciut M., Bran S., Ali S., Biris A.S. Novel multicomponent and biocompatible nanocomposite materials based on few-layer graphenes synthesized on a gold/hydroxyapatite catalytic system with applications in bone regeneration. J. Phys. Chem. C. 2011;115:18967–18976. [Google Scholar]
- 19.Zhu J., Wong H.M., Yeung K.W.K., Tjong S.C. Spark plasma sintered hydroxyapatite/graphite nanosheet and hydroxyapatite/multiwalled carbon nanotube composites: mechanical and in vitro cellular properties. Adv. Eng. Mater. 2011;13:336–341. [Google Scholar]
- 20.Marques P.A.A.P., Goncalves G., Singh M.K., Gracio J. Graphene oxide and hydroxyapatite as fillers of polylactic acid nanocomposites: preparation and characterization. J. Nanosci. Nanotechnol. 2012;12:6686–6692. doi: 10.1166/jnn.2012.4565. [DOI] [PubMed] [Google Scholar]
- 21.Marques P.A., Gonçalves G., Singh M.K., Grácio J. Graphene oxide and hydroxyapatite as fillers of polylactic acid nanocomposites: preparation and characterization. J. Nanosci. Nanotechnol. 2012;12:6686–6692. doi: 10.1166/jnn.2012.4565. [DOI] [PubMed] [Google Scholar]
- 22.Gonçalves G., Cruz S.M., Grácio J., Marques P.A., Ramírez-Santillán C., Vallet-Regí M., Portolés M.-T. New bioactive PMMA-hydroxyapatite based bone cement reinforced with graphene oxide. Graphene. 2012;2012 April 10–13, Bruselas, Belgica. [Google Scholar]
- 23.Ma H.B., Su W.X., Tai Z.X., Sun D.F., Yan X.B., Liu B., Xue Q.J. Preparation and cytocompatibility of polylactic acid/hydroxyapatite/graphene oxide nanocomposite fibrous membrane. Chin. Sci. Bull. 2012;57:3051–3058. [Google Scholar]
- 24.Liu H., Xi P., Xie G., Shi Y., Hou F., Huang L., Chen F., Zeng Z., Shao C., Wang J. Simultaneous reduction and surface functionalization of graphene oxide for hydroxyapatite mineralization. J. Phys. Chem. C. 2012;116:3334–3341. [Google Scholar]
- 25.Zhang L., Liu W., Yue C., Zhang T., Li P., Xing Z., Chen Y. A tough graphene nanosheet/hydroxyapatite composite with improved in vitro biocompatibility. Carbon. 2013;61:105–115. [Google Scholar]
- 26.Fan Z., Wang J., Wang Z., Li Z., Qiu Y., Wang H., Xu Y., Niu L., Gong P., Yang S. Casein phosphopeptide-biofunctionalized graphene biocomposite for hydroxyapatite biomimetic mineralization. J. Phys. Chem. C. 2013;117:10375–10382. [Google Scholar]
- 27.Li M., Liu Q., Jia Z., Xu X., Shi Y., Cheng Y., Zheng Y., Xi T., Wei S. Electrophoretic deposition and electrochemical behavior of novel graphene oxide-hyaluronic acid-hydroxyapatite nanocomposite coatings. Appl. Surf. Sci. 2013;284:804–810. [Google Scholar]
- 28.Zanin H., Saito E., Marciano F.R., Ceragioli H.J., Campos Granato A.E., Porcionatto M., Lobo A.O. Fast preparation of nano-hydroxyapatite/superhydrophilic reduced graphene oxide composites for bioactive applications. J. Mater. Chem. B. 2013;1:4947. doi: 10.1039/c3tb20550a. [DOI] [PubMed] [Google Scholar]
- 29.Neelgund G.M., Oki A., Luo Z. In situ deposition of hydroxyapatite on graphene nanosheets. Mater. Res. Bull. 2013;48:175–179. doi: 10.1016/j.materresbull.2012.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M., Wang Y., Liu Q., Li Q., Cheng Y., Zheng Y., Xi T., Wei S. In situ synthesis and biocompatibility of nano hydroxyapatite on pristine and chitosan functionalized graphene oxide. J. Mater. Chem. B. 2013;1:475–484. doi: 10.1039/c2tb00053a. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Y., Sun K.-N., Wang W.-L., Wang Y.-X., Sun X.-L., Liang Y.-J., Sun X.-N., Chui P.-F. Microstructure and anisotropic mechanical properties of graphene nanoplatelet toughened biphasic calcium phosphate composite. Ceram. Int. 2013;39:7627–7634. [Google Scholar]
- 32.Liu Y., Huang J., Li H. Synthesis of hydroxyapatite–reduced graphite oxide nanocomposites for biomedical applications: oriented nucleation and epitaxial growth of hydroxyapatite. J. Mater. Chem. B. 2013;1:1826. doi: 10.1039/c3tb00531c. [DOI] [PubMed] [Google Scholar]
- 33.Wang J., Wang H., Wang Y., Li J., Su Z., Wei G. Alternate layer-by-layer assembly of graphene oxide nanosheets and fibrinogen nanofibers on a silicon substrate for a biomimetic three-dimensional hydroxyapatite scaffold. J. Mater. Chem. B. 2014;2:7360–7368. doi: 10.1039/c4tb01324g. [DOI] [PubMed] [Google Scholar]
- 34.Ramani D., Sastry T.P. Bacterial cellulose-reinforced hydroxyapatite functionalized graphene oxide: a potential osteoinductive composite. Cellulose. 2014;21:3585–3595. [Google Scholar]
- 35.Liu H., Cheng J., Chen F., Hou F., Bai D., Xi P., Zeng Z. Biomimetic and cell-mediated mineralization of hydroxyapatite by carrageenan functionalized graphene oxide. ACS Appl. Mater. Interfaces. 2014;6:3132–3140. doi: 10.1021/am4057826. [DOI] [PubMed] [Google Scholar]
- 36.Mohandes F., Salavati-Niasari M. Freeze-drying synthesis, characterization and in vitro bioactivity of chitosan/graphene oxide/hydroxyapatite nanocomposite. Rsc Adv. 2014;4:25993–26001. [Google Scholar]
- 37.Liu H., Cheng J., Chen F., Bai D., Shao C., Wang J., Xi P., Zeng Z. Gelatin functionalized graphene oxide for mineralization of hydroxyapatite: biomimetic and in vitro evaluation. Nanoscale. 2014;6:5315–5322. doi: 10.1039/c4nr00355a. [DOI] [PubMed] [Google Scholar]
- 38.Li M., Liu Q., Jia Z., Xu X., Cheng Y., Zheng Y., Xi T., Wei S. Graphene oxide/hydroxyapatite composite coatings fabricated by electrophoretic nanotechnology for biological applications. Carbon. 2014;67:185–197. [Google Scholar]
- 39.Oyefusi A., Olanipekun O., Neelgund G.M., Peterson D., Stone J.M., Williams E., Carson L., Regisford G., Oki A. Hydroxyapatite grafted carbon nanotubes and graphene nanosheets: promising bone implant materials. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014;132:410–416. doi: 10.1016/j.saa.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y., Dang Z., Wang Y., Huang J., Li H. Hydroxyapatite/graphene-nanosheet composite coatings deposited by vacuum cold spraying for biomedical applications: inherited nanostructures and enhanced properties. Carbon. 2014;67:250–259. [Google Scholar]
- 41.Rodríguez-González C., Cid-Luna H.E., Salas P., Castaño V.M. Hydroxyapatite-functionalized graphene: a new hybrid nanomaterial. J. Nanomater. 2014;2014:1–7. [Google Scholar]
- 42.Mohandes F., Salavati-Niasari M. In vitro comparative study of pure hydroxyapatite nanorods and novel polyethylene glycol/graphene oxide/hydroxyapatite nanocomposite. J. Nanoparticle Res. 2014;16 [Google Scholar]
- 43.Núñez J.D., Benito A.M., González R., Aragón J., Arenal R., Maser W.K. Integration and bioactivity of hydroxyapatite grown on carbon nanotubes and graphene oxide. Carbon. 2014;79:590–604. [Google Scholar]
- 44.Baradaran S., Moghaddam E., Basirun W.J., Mehrali M., Sookhakian M., Hamdi M., Moghaddam M.R.N., Alias Y. Mechanical properties and biomedical applications of a nanotube hydroxyapatite-reduced graphene oxide composite. Carbon. 2014;69:32–45. [Google Scholar]
- 45.Zhao J., Zhang Z., Yu Z., He Z., Yang S., Jiang H. Nucleation and characterization of hydroxyapatite on thioglycolic acid-capped reduced graphene oxide/silver nanoparticles in simplified simulated body fluid. Appl. Surf. Sci. 2014;289:89–96. [Google Scholar]
- 46.Fan Z., Wang J., Wang Z., Ran H., Li Y., Niu L., Gong P., Liu B., Yang S. One-pot synthesis of graphene/hydroxyapatite nanorod composite for tissue engineering. Carbon. 2014;66:407–416. [Google Scholar]
- 47.Tatavarty R., Ding H., Lu G., Taylor R.J., Bi X. Synergistic acceleration in the osteogenesis of human mesenchymal stem cells by graphene oxide-calcium phosphate nanocomposites. Chem. Commun. (Camb) 2014;50:8484–8487. doi: 10.1039/c4cc02442g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao J., Zhang Z., Yu Z., Yang S., Jiang H. Synthesis of hydroxyapatite on thioglycolic acid-capped reduced graphene oxide/silver nanoparticles: effect of reaction condition in normal or pathological simulated body fluid. Mater. Lett. 2014;116:359–362. [Google Scholar]
- 49.Depan D., Pesacreta T.C., Misra R.D.K. The synergistic effect of a hybrid graphene oxide–chitosan system and biomimetic mineralization on osteoblast functions. Biomed. Sci. 2014;2:264–274. doi: 10.1039/c3bm60192g. [DOI] [PubMed] [Google Scholar]
- 50.Lavanya N., Sudhan N., Kanchana P., Radhakrishnan S., Sekar C. A new strategy for simultaneous determination of 4-aminophenol, uric acid and nitrite based on a graphene/hydroxyapatite composite modified glassy carbon electrode. RSC Adv. 2015;5:52703–52709. [Google Scholar]
- 51.Zanin H., Rosa C.M., Eliaz N., May P.W., Marciano F.R., Lobo A.O. Assisted deposition of nano-hydroxyapatite onto exfoliated carbon nanotube oxide scaffolds. Nanoscale. 2015;7:10218–10232. doi: 10.1039/c4nr07317g. [DOI] [PubMed] [Google Scholar]
- 52.Azhari A., Toyserkani E., Villain C. Additive manufacturing of graphene-hydroxyapatite nanocomposite structures. Int. J. Appl. Ceram. Technol. 2015;12:8–17. [Google Scholar]
- 53.Janković A., Eraković S., Mitrić M., Matić I.Z., Juranić Z.D., Tsui G.C.P., Tang C.-y., Mišković-Stanković V., Rhee K.Y., Park S.J. Bioactive hydroxyapatite/graphene composite coating and its corrosion stability in simulated body fluid. J. Alloy. Comp. 2015;624:148–157. [Google Scholar]
- 54.Gao F., Xu C., Hu H., Wang Q., Gao Y., Chen H., Guo Q., Chen D., Eder D. Biomimetic synthesis and characterization of hydroxyapatite/graphene oxide hybrid coating on Mg alloy with enhanced corrosion resistance. Mater. Lett. 2015;138:25–28. [Google Scholar]
- 55.Zakharov N.A., Ezhova Z.A., Koval E.M., Tkachev A.G., Kuznetsov N.T. Calcium hydroxyapatite in hydroxyapatite/graphene oxide/collagen nanohybrids. Russ. J. Inorg. Chem. 2015;60:1467–1480. [Google Scholar]
- 56.Baradaran S., Moghaddam E., Nasiri-Tabrizi B., Basirun W.J., Mehrali M., Sookhakian M., Hamdi M., Alias Y. Characterization of nickel-doped biphasic calcium phosphate/graphene nanoplatelet composites for biomedical application. Mater. Sci. Eng. C, Mater. Biol. Appl. 2015;49:656–668. doi: 10.1016/j.msec.2015.01.050. [DOI] [PubMed] [Google Scholar]
- 57.Ravichandran Y.D., Villaret T., Rajesh R. Development of a tricomponent composite graphene oxide-chitosan-hydroxyapatite for bone tissue engineering. J. Indian Chem. Soc. 2015;92:649–651. [Google Scholar]
- 58.Rajesh R., Ravichandran Y.D. Development of new graphene oxide incorporated tricomponent scaffolds with polysaccharides and hydroxyapatite and study of their osteoconductivity on MG-63 cell line for bone tissue engineering. RSC Adv. 2015;5:41135–41143. [Google Scholar]
- 59.Bajpai I., Kim D.-Y., Han Y.-H., Jang B.-K., Kim S. Directional property evaluation of spark plasma sintered GNPs-reinforced hydroxyapatite composites. Mater. Lett. 2015;158:62–65. [Google Scholar]
- 60.Bharath G., Veeramani V., Chen S.-M., Madhu R., Manivel Raja M., Balamurugan A., Mangalaraj D., Viswanathan C., Ponpandian N. Edge-carboxylated graphene anchoring magnetite-hydroxyapatite nanocomposite for an efficient 4-nitrophenol sensor. RSC Adv. 2015;5:13392–13401. [Google Scholar]
- 61.Sava S., Moldovan M., Sarosi C., Mesaros A., Dudea D., Alb C. Effects of graphene addition on the mechanical properties of composites for dental restoration. Mater. Plast. 2015;52:90–92. [Google Scholar]
- 62.Hong-Pei L., Ying-Bo W., Zhi S., Xiong L., Shuai W. Electrospinning gelatin/chitosan/hydroxyapatite/graphene oxide composite nanofibers with antibacterial properties. J. Inorg. Mater. 2015;30:516–522. [Google Scholar]
- 63.Lee J.H., Shin Y.C., Lee S.M., Jin O.S., Kang S.H., Hong S.W., Jeong C.M., Huh J.B., Han D.W. Enhanced osteogenesis by reduced graphene oxide/hydroxyapatite nanocomposites. Sci. Rep. 2015;5:18833. doi: 10.1038/srep18833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bharath G., Madhu R., Chen S.-M., Veeramani V., Balamurugan A., Mangalaraj D., Viswanathan C., Ponpandian N. Enzymatic electrochemical glucose biosensors by mesoporous 1D hydroxyapatite-on-2D reduced graphene oxide. J. Mater. Chem. B. 2015;3:1360–1370. doi: 10.1039/c4tb01651c. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Q., Liu Y., Zhang Y., Li H., Tan Y., Luo L., Duan J., Li K., Banks C.E. Facile and controllable synthesis of hydroxyapatite/graphene hybrid materials with enhanced sensing performance towards ammonia. Analyst. 2015;140:5235–5242. doi: 10.1039/c5an00622h. [DOI] [PubMed] [Google Scholar]
- 66.Xie X., Hu K., Fang D., Shang L., Tran S.D., Cerruti M. Graphene and hydroxyapatite self-assemble into homogeneous, free standing nanocomposite hydrogels for bone tissue engineering. Nanoscale. 2015;7:7992–8002. doi: 10.1039/c5nr01107h. [DOI] [PubMed] [Google Scholar]
- 67.Janković A., Eraković S., Vukašinović-Sekulić M., Mišković-Stanković V., Park S.J., Rhee K.Y. Graphene-based antibacterial composite coatings electrodeposited on titanium for biomedical applications. Prog. Org. Coating. 2015;83:1–10. [Google Scholar]
- 68.Nair M., Nancy D., Krishnan A.G., Anjusree G.S., Vadukumpully S., Nair S.V. Graphene oxide nanoflakes incorporated gelatin-hydroxyapatite scaffolds enhance osteogenic differentiation of human mesenchymal stem cells. Nanotechnology. 2015;26:161001. doi: 10.1088/0957-4484/26/16/161001. [DOI] [PubMed] [Google Scholar]
- 69.Wu C., Xia L., Han P., Xu M., Fang B., Wang J., Chang J., Xiao Y. Graphene-oxide-modified β-tricalcium phosphate bioceramics stimulate in vitro and in vivo osteogenesis. Carbon. 2015;93:116–129. [Google Scholar]
- 70.Mahto T.K., Chandra Pandey S., Chandra S., Kumar A., Sahu S.k. Hydroxyapatite conjugated graphene oxide nanocomposite: a new sight for significant applications in adsorption. RSC Adv. 2015;5:96313–96322. [Google Scholar]
- 71.Yan Y., Zhang X., Mao H., Huang Y., Ding Q., Pang X. Hydroxyapatite/gelatin functionalized graphene oxide composite coatings deposited on TiO2 nanotube by electrochemical deposition for biomedical applications. Appl. Surf. Sci. 2015;329:76–82. [Google Scholar]
- 72.Crisan L., Crisan B., Soritau O., Baciut M., Biris A.R., Baciut G., Lucaciu O. In vitro study of biocompatibility of a graphene composite with gold nanoparticles and hydroxyapatite on human osteoblasts. J. Appl. Toxicol. 2015;35:1200–1210. doi: 10.1002/jat.3152. [DOI] [PubMed] [Google Scholar]
- 73.Deepachitra R., Nigam R., Purohit S.D., Kumar B.S., Hemalatha T., Sastry T.P. In vitrostudy of hydroxyapatite coatings on fibrin functionalized/pristine graphene oxide for bone grafting. Mater. Manuf. Process. 2014;30:804–811. [Google Scholar]
- 74.Cheng J., Liu H., Zhao B., Shen R., Liu D., Hong J., Wei H., Xi P., Chen F., Bai D. MC3T3-E1 preosteoblast cell-mediated mineralization of hydroxyapatite by poly-dopamine-functionalized graphene oxide. J. Bioact. Compat. Polym. 2015;30:289–301. [Google Scholar]
- 75.Cui L., Wang Y., Hu L., Gao L., Du B., Wei Q. Mechanism of Pb(ii) and methylene blue adsorption onto magnetic carbonate hydroxyapatite/graphene oxide. RSC Adv. 2015;5:9759–9770. [Google Scholar]
- 76.Xiong G., Luo H., Zuo G., Ren K., Wan Y. Novel porous graphene oxide and hydroxyapatite nanosheets-reinforced sodium alginate hybrid nanocomposites for medical applications. Mater. Char. 2015;107:419–425. [Google Scholar]
- 77.Lee J.H., Shin Y.C., Jin O.S., Kang S.H., Hwang Y.S., Park J.C., Hong S.W., Han D.W. Reduced graphene oxide-coated hydroxyapatite composites stimulate spontaneous osteogenic differentiation of human mesenchymal stem cells. Nanoscale. 2015;7:11642–11651. doi: 10.1039/c5nr01580d. [DOI] [PubMed] [Google Scholar]
- 78.Wang J., Ouyang Z., Ren Z., Li J., Zhang P., Wei G., Su Z. Self-assembled peptide nanofibers on graphene oxide as a novel nanohybrid for biomimetic mineralization of hydroxyapatite. Carbon. 2015;89:20–30. [Google Scholar]
- 79.Klébert S., Balázsi C., Balázsi K., Bódis E., Fazekas P., Keszler A.M., Szépvölgyi J., Károly Z. Spark plasma sintering of graphene reinforced hydroxyapatite composites. Ceram. Int. 2015;41:3647–3652. [Google Scholar]
- 80.Shin Y.C., Lee J.H., Jin O.S., Kang S.H., Hong S.W., Kim B., Park J.-C., Han D.-W. Synergistic effects of reduced graphene oxide and hydroxyapatite on osteogenic differentiation of MC3T3-E1 preosteoblasts. Carbon. 2015;95:1051–1060. [Google Scholar]
- 81.Zakharov N.A., Ezhova Z.A., Koval E.M., Kalinnikov V.T., Tkachev A.G. Synthesis and physicochemical characteristics of the calcium hydroxyapatite/graphene oxide hybrid nanocomposite. Russ. J. Inorg. Chem. 2015;60:804–816. [Google Scholar]
- 82.Zhao C., Lu X., Zanden C., Liu J. The promising application of graphene oxide as coating materials in orthopedic implants: preparation, characterization and cell behavior. Biomed. Mater. 2015;10:015019. doi: 10.1088/1748-6041/10/1/015019. [DOI] [PubMed] [Google Scholar]
- 83.Bai Y., Bai Y., Gao J., Ma W., Su J., Jia R. Preparation and characterization of reduced graphene oxide/fluorhydroxyapatite composites for medical implants. J. Alloy. Comp. 2016;688:657–667. [Google Scholar]
- 84.Ettorre V., De Marco P., Zara S., Perrotti V., Scarano A., Di Crescenzo A., Petrini M., Hadad C., Bosco D., Zavan B., Valbonetti L., Spoto G., Iezzi G., Piattelli A., Cataldi A., Fontana A. In vitro and in vivo characterization of graphene oxide coated porcine bone granules. Carbon. 2016;103:291–298. [Google Scholar]
- 85.Fan Z., Wang J., Liu F., Nie Y., Ren L., Liu B. A new composite scaffold of bioactive glass nanoparticles/graphene: synchronous improvements of cytocompatibility and mechanical property. Colloids Surf. B Biointerfaces. 2016;145:438–446. doi: 10.1016/j.colsurfb.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 86.Feng P., Peng S., Wu P., Gao C., Huang W., Deng Y., Xiao T., Shuai C. A nano-sandwich construct built with graphene nanosheets and carbon nanotubes enhances mechanical properties of hydroxyapatite-polyetheretherketone scaffolds. Int. J. Nanomed. 2016;11:3487–3500. doi: 10.2147/IJN.S110920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hermenean A., Dinescu S., Ionita M., Costache M. 2016. The Impact of Graphene Oxide on Bone Regeneration Therapies. [Google Scholar]
- 88.Li M., Xiong P., Mo M., Cheng Y., Zheng Y. Electrophoretic-deposited novel ternary silk fibroin/graphene oxide/hydroxyapatite nanocomposite coatings on titanium substrate for orthopedic applications. Front. Mater. Sci. 2016;10:270–280. [Google Scholar]
- 89.Liu C., Wong H.M., Yeung K.W.K., Tjong S.C. Novel electrospun polylactic acid nanocomposite fiber mats with hybrid graphene oxide and nanohydroxyapatite reinforcements having enhanced biocompatibility. Polymers. 2016;8 doi: 10.3390/polym8080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu Y., Huang J., Niinomi M., Li H. Inhibited grain growth in hydroxyapatite–graphene nanocomposites during high temperature treatment and their enhanced mechanical properties. Ceram. Int. 2016;42:11248–11255. [Google Scholar]
- 91.Metoki N., Rosa C., Zanin H., Marciano F., Eliaz N., Lobo A. Electrodeposition and biomineralization of nano-β-tricalcium phosphate on graphenated carbon nanotubes. Surf. Coating. Technol. 2016;297:51–57. [Google Scholar]
- 92.Mo X., Wei Y., Zhang X., Cai Q., Shen Y., Dai X., Meng S., Liu X., Liu Y., Hu Z., Deng X. Enhanced stem cell osteogenic differentiation by bioactive glass functionalized graphene oxide substrates. J. Nanomater. 2016;2016:1–11. Article ID 5613980. [Google Scholar]
- 93.Neelgund G.M., Oki A.R. Influence of carbon nanotubes and graphene nanosheets on photothermal effect of hydroxyapatite. J. Colloid Interface Sci. 2016;484:135–145. doi: 10.1016/j.jcis.2016.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prabhu S.M., Elanchezhiyan S.S., Lee G., Khan A., Meenakshi S. Assembly of nano-sized hydroxyapatite onto graphene oxide sheets via in-situ fabrication method and its prospective application for defluoridation studies. Chem. Eng. J. 2016;300:334–342. [Google Scholar]
- 95.Raja C.A., Balakumar S., Durgalakshmi D., George R.P., Anandkumar B., Mudali U.K. Reduced graphene oxide/nano-Bioglass composites: processing and super-anion oxide evaluation. Rsc Adv.s. 2016;6:19657–19661. [Google Scholar]
- 96.Raucci M.G., Giugliano D., Longo A., Zeppetelli S., Carotenuto G., Ambrosio L. Comparative facile methods for preparing graphene oxide-hydroxyapatite for bone tissue engineering. J. Tissue Eng. Regen. Med. August 2017;11(8):2204–2216. doi: 10.1002/term.2119. [DOI] [PubMed] [Google Scholar]
- 97.Rodrigues B.V., Leite N.C., Cavalcanti B.D.N., Silva N.S.D., Marciano F.R., Corat E.J., Webster T.J., Lobo A.O. Graphene oxide/multi-walled carbon nanotubes as nano featured scaffolds for the assisted deposition of nanohydroxyapatite: characterization and biological evaluation. Int. J. Nanomed. 2016;11:2569–2585. doi: 10.2147/IJN.S106339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shadjou N., Hasanzadeh M. Graphene and its nanostructure derivatives for use in bone tissue engineering: recent advances. J. Biomed. Mater. Res. Part A. 2016;104:1250–1275. doi: 10.1002/jbm.a.35645. [DOI] [PubMed] [Google Scholar]
- 99.Shao W., He J., Wang Q., Cui S., Ding B. Biomineralized poly (L-lactic-co-glycolic acid)/graphene oxide/Tussah silk fibroin nanofiber scaffolds with multiple orthogonal layers enhance osteoblastic differentiation of mesenchymal stem cells. ACS Biomater. Sci. Eng. 2017;3(7):1370–1380. doi: 10.1021/acsbiomaterials.6b00533. [DOI] [PubMed] [Google Scholar]
- 100.Shi Y.Y., Li M., Liu Q., Jia Z.J., Xu X.C., Cheng Y., Zheng Y.F. Electrophoretic deposition of graphene oxide reinforced chitosan-hydroxyapatite nanocomposite coatings on Ti substrate. J. Mater. Sci. Mater. Med. 2016;27:48. doi: 10.1007/s10856-015-5634-9. [DOI] [PubMed] [Google Scholar]
- 101.Song F., Jie W., Zhang T., Li W., Jiang Y., Wan L., Liu W., Li X., Liu B. Room-temperature fabrication of a three-dimensional reduced-graphene oxide/polypyrrole/hydroxyapatite composite scaffold for bone tissue engineering. RSC Adv. 2016;6:92804–92812. [Google Scholar]
- 102.Spanos K.N., Anifantis N.K. Finite element prediction of stress transfer in graphene nanocomposites: the interface effect. Compos. Struct. 2016;154:269–276. [Google Scholar]
- 103.Tavafoghi M., Brodusch N., Gauvin R., Cerruti M. Hydroxyapatite formation on graphene oxide modified with amino acids: arginine versus glutamic acid. J. R. Soc. Interface/R. Soc. 2016;13:20150986. doi: 10.1098/rsif.2015.0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xie C., Lu X., Han L., Xu J., Wang Z., Jiang L., Wang K., Zhang H., Ren F., Tang Y. Biomimetic mineralized hierarchical graphene oxide/chitosan scaffolds with adsorbability for immobilization of nanoparticles for biomedical applications. ACS Appl. Mater. Interfaces. 2016;8:1707–1717. doi: 10.1021/acsami.5b09232. [DOI] [PubMed] [Google Scholar]
- 105.Zakharov N.A., Tkachev A.G., Demina L.I., Kiselev M.R., Kalinnikov V.T. The effect of graphene oxide (GO) on biomineralization and solubility of calcium hydroxyapatite (HA) Protect. Met. Phys. Chem. Surf. 2016;52:665–676. [Google Scholar]
- 106.Zeng Y., Pei X., Yang S., Qin H., Cai H., Hu S., Sui L., Wan Q., Wang J. Graphene oxide/hydroxyapatite composite coatings fabricated by electrochemical deposition. Surf. Coating. Technol. 2016;286:72–79. [Google Scholar]
- 107.Zhang J., Zhu F., Zhang Y., Hu M., Chi Y., Zhang X., Guo X. In vitro bioactivity, degradation property and cell viability of the CaP/chitosan/graphene coating on magnesium alloy in m-SBF. Int. J. Electrochem. Sci. 2016;11:9326–9339. [Google Scholar]
- 108.Zhang R., Metoki N., Sharabani-Yosef O., Zhu H., Eliaz N. Hydroxyapatite/mesoporous graphene/single-walled carbon nanotubes freestanding flexible hybrid membranes for regenerative medicine. Adv. Funct. Mater. 2016;26:7965–7974. [Google Scholar]
- 109.Zhang R., Metoki N., Sharabani-Yosef O., Zhu H., Eliaz N. Cycling-stable cathodes: hydroxyapatite/mesoporous graphene/single-walled carbon nanotubes freestanding flexible hybrid membranes for regenerative medicine (Adv. Funct. Mater. 44/2016) Adv. Funct. Mater. 2016;26 7946–7946. [Google Scholar]
- 110.Zhang S., Yang Q., Zhao W., Qiao B., Cui H., Fan J., Li H., Tu X., Jiang D. In vitro and in vivo biocompatibility and osteogenesis of graphene-reinforced nanohydroxyapatite polyamide66 ternary biocomposite as orthopedic implant material. Int. J. Nanomed. 2016;11:3179–3189. doi: 10.2147/IJN.S105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang T., Li N., Li K., Gao R., Gu W., Wu C., Su R., Liu L., Zhang Q., Liu J. Enhanced proliferation and osteogenic differentiation of human mesenchymal stem cells on biomineralized three-dimensional graphene foams. Carbon. 2016;105:233–243. [Google Scholar]
- 112.Zheng J., Xiao W., Fan Y., Xu X., Zhang K., Xie D., Luo R., Yang X., Chen B. Electro-deposited calcium phosphate compounds on graphene sheets: blossoming flowers. Mater. Lett. 2016;179:122–125. [Google Scholar]
- 113.Anirudhan T.S., Deepa J.R., Nair A.S. Fabrication of chemically modified graphene oxide/nano hydroxyapatite composite for adsorption and subsequent photocatalytic degradation of aureomycine hydrochloride. J. Ind. Eng. Chem. 2017;47:415–430. [Google Scholar]
- 114.Azhari A. 2017. Additive Manufacturing of Graphene-based Devices. [Google Scholar]
- 115.Bharath G., Latha B.S., Alsharaeh E.H., Prakash P., Ponpandian N. Enhanced hydroxyapatite nanorods formation on graphene oxide nanocomposite as a potential candidate for protein adsorption, pH controlled release and an effective drug delivery platform for cancer therapy. Anal. Methods. 2017;9:240–252. [Google Scholar]
- 116.Đošić M., Eraković S., Janković A., Vukašinović-Sekulić M., Matić I.Z., Stojanović J., Rhee K.Y., Mišković-Stanković V., Park S.-J. In vitro investigation of electrophoretically deposited bioactive hydroxyapatite/chitosan coatings reinforced by graphene. J. Ind. Eng. Chem. 2017;47:336–347. [Google Scholar]
- 117.Eqtesadi S., Motealleh A., Wendelbo R., Ortiz A.L., Miranda P. Reinforcement with reduced graphene oxide of bioactive glass scaffolds fabricated by robocasting. J. Eur. Ceram. Soc. 2017;37:3695–3704. [Google Scholar]
- 118.Holt B.D., Wright Z.M., Arnold A.M., Sydlik S.A. Graphene oxide as a scaffold for bone regeneration. Wiley Interdiscipl. Rev. Nanomed. Nanobiotechnol. 2017;9 doi: 10.1002/wnan.1437. [DOI] [PubMed] [Google Scholar]
- 119.Huang J., Gong Y., Liu Y., Suo X., Li H. Developing titania-hydroxyapatite-reduced graphene oxide nanocomposite coatings by liquid flame spray deposition for photocatalytic applications. J. Eur. Ceram. Soc. 2017;37:3705–3711. [Google Scholar]
- 120.Jakus A.E., Shah R.N. Multi and mixed 3D-printing of graphene-hydroxyapatite hybrid materials for complex tissue engineering. J. Biomed. Mater. Res. Part A. 2017;105:274–283. doi: 10.1002/jbm.a.35684. [DOI] [PubMed] [Google Scholar]
- 121.Li Z., Khun N.W., Tang X.Z., Liu E., Khor K.A. Mechanical, tribological and biological properties of novel 45S5 Bioglass(R) composites reinforced with in situ reduced graphene oxide. J. Mech. Behav. Biomed. Mater. 2017;65:77–89. doi: 10.1016/j.jmbbm.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 122.Nie W., Peng C., Zhou X., Chen L., Wang W., Zhang Y., Ma P.X., He C. Three-dimensional porous scaffold by self-assembly of reduced graphene oxide and nano-hydroxyapatite composites for bone tissue engineering. Carbon. 2017;116:325–337. [Google Scholar]
- 123.Pulyala P., Singh A., Dias-Netipanyj M.F., Cogo S.C., Santos L.S., Soares P., Gopal V., Suganthan V., Manivasagam G., Popat K.C. In-vitro cell adhesion and proliferation of adipose derived stem cell on hydroxyapatite composite surfaces. Mater. Sci. Eng. C, Mater. Biol. Appl. 2017;75:1305–1316. doi: 10.1016/j.msec.2017.02.175. [DOI] [PubMed] [Google Scholar]
- 124.Rajesh A., Mangamma G., Sairam T.N., Subramanian S., Kalavathi S., Kamruddin M., Dash S. Physicochemical properties of nanocomposite: hydroxyapatite in reduced graphene oxide. Mater. Sci. Eng. C, Mater. Biol. Appl. 2017;76:203–210. doi: 10.1016/j.msec.2017.02.044. [DOI] [PubMed] [Google Scholar]
- 125.Sricharoen P., Limchoowong N., Areerob Y., Nuengmatcha P., Techawongstien S., Chanthai S. Fe3O4/hydroxyapatite/graphene quantum dots as a novel nano-sorbent for preconcentration of copper residue in Thai food ingredients: optimization of ultrasound-assisted magnetic solid phase extraction. Ultrason. Sonochem. 2017;37:83–93. doi: 10.1016/j.ultsonch.2016.12.037. [DOI] [PubMed] [Google Scholar]
- 126.Wen C., Zhan X., Huang X., Xu F., Luo L., Xia C. Characterization and corrosion properties of hydroxyapatite/graphene oxide bio-composite coating on magnesium alloy by one-step micro-arc oxidation method. Surf. Coating. Technol. 2017;317:125–133. [Google Scholar]
- 127.Weng W., Nie W., Zhou Q., Zhou X., Cao L., Ji F., Cui J., He C., Su J. Controlled release of vancomycin from 3D porous graphene-based composites for dual-purpose treatment of infected bone defects. RSC Adv. 2017;7:2753–2765. [Google Scholar]
- 128.Yao C., Zhu J., Xie A., Shen Y., Li H., Zheng B., Wei Y. Graphene oxide and creatine phosphate disodium dual template-directed synthesis of GO/hydroxyapatite and its application in drug delivery. Mater. Sci. Eng. C, Mater. Biol. Appl. 2017;73:709–715. doi: 10.1016/j.msec.2016.11.083. [DOI] [PubMed] [Google Scholar]
- 129.Yu P., Bao R.-Y., Shi X.-J., Yang W., Yang M.-B. Self-assembled high-strength hydroxyapatite/graphene oxide/chitosan composite hydrogel for bone tissue engineering. Carbohydr. Polym. 2017;155:507–515. doi: 10.1016/j.carbpol.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 130.Zhou K., Gao R., Jiang S. Morphology, thermal and mechanical properties of poly (epsilon-caprolactone) biocomposites reinforced with nano-hydroxyapatite decorated graphene. J. Colloid Interface Sci. 2017;496:334–342. doi: 10.1016/j.jcis.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 131.Depan D., Pesacreta T.C., Misra R.D. The synergistic effect of a hybrid graphene oxide-chitosan system and biomimetic mineralization on osteoblast functions. Biomed. Sci. 2013;2:264–274. doi: 10.1039/c3bm60192g. [DOI] [PubMed] [Google Scholar]
- 132.Zhang Y., Zhang L.Y., Zhou C.W. Review of chemical vapor deposition of graphene and related applications. Accounts Chem. Res. 2013;46:2329–2339. doi: 10.1021/ar300203n. [DOI] [PubMed] [Google Scholar]
- 133.Gu Y.W., Loh N.H., Khor K.A., Tor S.B., Cheang P. Spark plasma sintering of hydroxyapatite powders. Biomaterials. 2002;23:37–43. doi: 10.1016/s0142-9612(01)00076-x. [DOI] [PubMed] [Google Scholar]
- 134.Atkinson H.V., Davies S. Fundamental aspects of hot isostatic pressing: an overview. Metall. Mater. Trans. A. 2000;31:2981–3000. [Google Scholar]
- 135.Boilet L., Descamps M., Rguiti E., Tricoteaux A., Lu J.X., Petit F., Lardot V., Cambier F., Leriche A. Processing and properties of transparent hydroxyapatite and beta tricalcium phosphate obtained by HIP process. Ceram. Int. 2013;39:283–288. [Google Scholar]
- 136.Boccaccini A.R., Keim S., Ma R., Li Y., Zhitomirsky I. Electrophoretic deposition of biomaterials. J. R. Soc. Interface. 2010;7:S581–S613. doi: 10.1098/rsif.2010.0156.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chavez-Valdez A., Shaffer M.S.P., Boccaccini A.R. Applications of graphene electrophoretic deposition. A review. J. Phys. Chem. B. 2013;117:1502–1515. doi: 10.1021/jp3064917. [DOI] [PubMed] [Google Scholar]
- 138.Cote L.J., Kim J., Tung V.C., Luo J.Y., Kim F., Huang J.X. Graphene oxide as surfactant sheets. Pure Appl. Chem. 2011;83:95–110. [Google Scholar]
- 139.Zhao H.X., Dong W.J., Zheng Y.Y., Liu A.P., Yao J.M., Li C.R., Tang W.H., Chen B.Y., Wang G., Shi Z. The structural and biological properties of hydroxyapatite-modified titanate nanowire scaffolds. Biomaterials. 2011;32:5837–5846. doi: 10.1016/j.biomaterials.2011.04.083. [DOI] [PubMed] [Google Scholar]
- 140.Fu C., Song B.A., Wan C.Y., Savino K., Wang Y., Zhang X.Y., Yates M.Z. Electrochemical growth of composite hydroxyapatite coatings for controlled release. Surf. Coating. Technol. 2015;276:618–625. [Google Scholar]
- 141.Berndt C.C., Hasan M.F., Tietz U., Schmitz K.P. Springer Berlin Heidelberg; 2014. A Review of Hydroxyapatite Coatings Manufactured by Thermal Spray. [Google Scholar]
- 142.Li N., Zhang Q., Gao S., Song Q., Huang R., Wang L., Liu L.W., Dai J.W., Tang M.L., Cheng G.S. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci. Rep.-Uk. 2013;3 doi: 10.1038/srep01604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lim H.N., Huang N.M., Lim S.S., Harrison I., Chia C.H. Fabrication and characterization of graphene hydrogel via hydrothermal approach as a scaffold for preliminary study of cell growth. Int. J. Nanomed. 2011;6:1817–1823. doi: 10.2147/IJN.S23392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Repanas A., Andriopoulou S., Glasmacher B. The significance of electrospinning as a method to create fibrous scaffolds for biomedical engineering and drug delivery applications. J. Drug Deliv. Sci. Technol. February 2016;31:137–146. [Google Scholar]
- 145.Bose S., Vahabzadeh S., Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater. Today. 2013;16:496–504. [Google Scholar]
- 146.Lee C., Wei X.D., Kysar J.W., Hone J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science. 2008;321:385–388. doi: 10.1126/science.1157996. [DOI] [PubMed] [Google Scholar]
- 147.Hao F., Fang D., Xu Z. Mechanical and thermal transport properties of graphene with defects. Appl. Phys. Lett. 2011;99 041901–041901-041903. [Google Scholar]
- 148.Terrones H., Lv R., Terrones M., Dresselhaus M.S. The role of defects and doping in 2D graphene sheets and 1D nanoribbons. Rep. Prog. Phys. 2012;75:062501. doi: 10.1088/0034-4885/75/6/062501. [DOI] [PubMed] [Google Scholar]
- 149.Dreyer D.R., Park S., Bielawski C.W., Ruoff R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2009;43:5288. doi: 10.1039/c4cs00060a. [DOI] [PubMed] [Google Scholar]
- 150.Suk J.W., Piner R.D., An J., Ruoff R.S. Mechanical properties of monolayer graphene oxide. Acs Nano. 2016;4:6557–6564. doi: 10.1021/nn101781v. [DOI] [PubMed] [Google Scholar]
- 151.Gómez-Navarro C., Burghard M., Kern K. Elastic properties of chemically derived single graphene sheets. Nano Lett. 2008;8:2045–2049. doi: 10.1021/nl801384y. [DOI] [PubMed] [Google Scholar]
- 152.Stankovich S., Dikin D.A., Dommett G.H., Kohlhaas K.M., Zimney E.J., Stach E.A., Piner R.D., Nguyen S.T., Ruoff R.S. Graphene-based composite materials. Nature. 2006;442:282. doi: 10.1038/nature04969. [DOI] [PubMed] [Google Scholar]
- 153.Liu J., Yan H., Jiang K. Mechanical properties of graphene platelet-reinforced alumina ceramic composites. Ceram. Int. 2013;39:6215–6221. [Google Scholar]
- 154.Liu Y., Huang J., Li H. Synthesis of hydroxyapatite-reduced graphite oxide nanocomposites for biomedical applications: oriented nucleation and epitaxial growth of hydroxyapatite. J. Mater. Chem. B. 2013;1:1826–1834. doi: 10.1039/c3tb00531c. [DOI] [PubMed] [Google Scholar]
- 155.Li Y.L., Liu C.L., Zhai H.L., Zhu G.X., Pan H.H., Xu X.R., Tang R.K. Biomimetic graphene oxide-hydroxyapatite composites via in situ mineralization and hierarchical assembly. Rsc Adv. 2014;4:25398–25403. [Google Scholar]
- 156.Astm S.F. 2008. Standard Practice for Assessment of Hemolytic Properties of Materials. Astm. [Google Scholar]
- 157.Liao K.H., Lin Y.S., Macosko C.W., Haynes C.L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. Acs Appl. Mater. Interfaces. 2011;3:2607. doi: 10.1021/am200428v. [DOI] [PubMed] [Google Scholar]
- 158.Kokubo T., Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27:2907–2915. doi: 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 159.Bohner M., Lemaitre J. Can bioactivity be tested in vitro with SBF solution? Biomaterials. 2009;30:2175–2179. doi: 10.1016/j.biomaterials.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 160.Lee W.C., Lim C.H., Shi H., Tang L.A., Wang Y., Lim C.T., Loh K.P. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. Acs Nano. 2011;5:7334–7341. doi: 10.1021/nn202190c. [DOI] [PubMed] [Google Scholar]
- 161.Jia Z., Xiu P., Li M., Xu X., Shi Y., Cheng Y., Wei S., Zheng Y., Xi T., Cai H. Bioinspired anchoring AgNPs onto micro-nanoporous TiO2 orthopedic coatings: trap-killing of bacteria, surface-regulated osteoblast functions and host responses. Biomaterials. 2015;75:203–222. doi: 10.1016/j.biomaterials.2015.10.035. [DOI] [PubMed] [Google Scholar]
- 162.Liu Y., Huang J., Ding S., Liu Y., Yuan J., Li H. Deposition, characterization, and enhanced adherence of escherichia coli bacteria on flame-sprayed photocatalytic titania-hydroxyapatite coatings. J. Therm. Spray Technol. 2013;22:1053–1062. [Google Scholar]
- 163.Hu W., Peng C., Luo W., Lv M., Li X., Li D., Huang Q., Fan C. Graphene-based antibacterial paper. Acs Nano. 2010;4:4317–4323. doi: 10.1021/nn101097v. [DOI] [PubMed] [Google Scholar]
- 164.Liu S., Zeng T.H., Hofmann M., Burcombe E., Wei J., Jiang R., Kong J., Chen Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. Acs Nano. 2011;5:6971–6980. doi: 10.1021/nn202451x. [DOI] [PubMed] [Google Scholar]
- 165.Akhavan O., Ghaderi E. Toxicity of graphene and graphene oxide nanowalls against bacteria. Acs Nano. 2010;4:5731. doi: 10.1021/nn101390x. [DOI] [PubMed] [Google Scholar]