Abstract
Background
This randomized controlled trial (RCT) will examine the efficacy of supervised functional electrical stimulation (FES) cycling on walking performance and physiological function among persons with multiple sclerosis (MS) with severe mobility disability.
Methods/design
This RCT will recruit 16 persons with MS that require unilateral or bilateral assistance for ambulation (i.e., Expanded Disability Status Scale (EDSS) score = 6.0–6.5). Participants will be randomized to one of two conditions: supervised FES cycling or passive cycling. The FES cycling condition will involve mild electrical stimulation that will generate an activation pattern that results in cycling the leg ergometer. The passive cycling condition will not provide any electrical stimulation, rather the movement of the pedals will be controlled by the electrical motor. Both conditions will be delivered 3 days/week for the same duration, over 6 months. Primary outcomes will include walking performance assessed as walking speed, endurance, and agility. Secondary outcomes will include physiological function assessed as cardiorespiratory fitness, muscular strength, and balance. Assessments will take place at baseline, mid-point (3-months), and immediately following the intervention (6-months).
Discussion
This study will lay the foundation for the design of a future RCT by: (1) providing effect sizes that can be included in a power analysis for optimal sample size estimation; and (2) identifying cardiorespiratory fitness, muscular strength, and balance (i.e., physiological function) as mechanisms for the beneficial effects of FES cycling on walking performance. This trial will provide important information on a novel exercise rehabilitation therapy for managing walking impairment in persons with severe MS.
Keywords: Multiple sclerosis, Randomized controlled trial, Exercise, Walking, Fitness
1. Introduction
Multiple sclerosis (MS) affects an estimated 1 per 1000 persons in the United States [1], [2]. The disease pathology initially involves immune-mediated demyelination and transection of axons within the central nervous system (CNS), and later transitions into a neurodegenerative process associated with axo-neuronal loss [3], [4], [5]. The extent and location of this damage within the CNS results in walking dysfunction [6] that can be exacerbated by physiological deconditioning or detraining brought about by physical inactivity [7], [8]. Exercise training is one approach for increasing physical activity, improving physiological function, and restoring walking performance in persons with MS [7], [8], [9].
Exercise training has a variety of beneficial effects for persons with MS [10], [11], [12]; however, the majority of previous research has been conducted in samples with mild or moderate disability levels (i.e., Expanded Disability Status Scale (EDSS) scores <6.0) rather than among those with severe ambulatory impairment [10], [13], [14]. This is critical considering long-standing arguments that rehabilitation, including exercise training, is the only practical means of preserving and improving functional outcomes in patients with severe MS [14], [15]. The provision of exercise training in persons with severe MS disability requires specialized training modalities [16], [17]. One such approach involves functional electrical stimulation (FES) cycling [16], [17].
FES cycling is an activity-based rehabilitation modality that involves transcutaneous electrical stimulation of leg muscles as an approach for producing leg-cycle ergometry. This method has been effective for improving walking performance and physiological function (e.g., cardiorespiratory fitness and muscular strength) in persons with incomplete spinal cord injury (SCI) and stroke [17], [18], [19]. Few studies, however, have examined the potential of FES cycling in persons with MS. One observational study has documented benefits of unsupervised FES cycling delivered over 6 months on walking speed and endurance, gait, and muscular strength in 5 persons with severe MS (mdn EDSS = 6.5) [20]. Two small (n = 8), uncontrolled studies involving 12–18 sessions of FES cycling demonstrated improved muscle metabolism [21] and increased thigh volume [22] in severe MS samples (EDSS > 6.0). FES cycling is an exercise rehabilitation approach that can be delivered in the home for potentially managing progressive mobility disability in severe MS. However, before home-based FES cycling can be pursued, we require high quality pilot data from a randomized trial with an appropriate control condition that demonstrates initial efficacy of this intervention.
This study involves a randomized controlled trial (RCT) for examining the efficacy of 6-months of supervised FES cycling versus a passive cycling control condition on walking performance and physiological function among persons with severe MS disability (i.e., EDSS = 6.0–6.5). This study will lay the foundation for the design of a future RCT by: (1) providing effect sizes that can be included in a power analysis for optimal sample size estimation; and (2) identifying cardiorespiratory fitness, muscular strength, and balance (i.e., physiological function) as mechanisms for the beneficial effects of FES cycling on walking performance.
2. Methods
2.1. Study design, overview and hypotheses
The proposed study will take place at the University of Illinois Urbana-Champaign campus. The study will involve a parallel group, assessor-blinded, RCT design for examining the efficacy of supervised FES cycling versus a passive cycling condition in patients with MS that require unilateral or bilateral assistance for ambulation. The primary outcome of the study will be walking performance assessed using the Timed 25-Foot Walk (T25FW), the 2-Minute Walk (2 MW), and the Timed Up-and-Go (TUG) tests. Secondary outcomes will involve measures of physiological function including cardiorespiratory fitness measured as peak oxygen consumption from an incremental exercise test, muscular strength assessed as peak torque of the knee flexors and extensors on a computerized dynamometer, and static balance assessed as center of pressure motion quantified with a force platform. We will recruit a sample of 16 persons with severe MS (i.e., EDSS score = 6.0–6.5). Participants who meet the eligibility criteria will be randomly allocated into either the FES cycling condition or the passive cycling condition. The FES cycling condition will involve mild electrical stimulation to the leg muscles that will generate an activation pattern that results in cycling the leg ergometer. The passive cycling condition will not provide any electrical stimulation to the leg muscles, rather the movement of the pedals will be controlled by the electrical motor. Both conditions will be delivered 3 days per week for the same duration, over 6 months using RT300 cycles (Restorative Therapies Inc, Baltimore, MD). Primary and secondary outcomes will be collected at baseline, 3-months, and 6-months. The effect of the intervention on primary and secondary outcomes will be examined using a Condition (FES cycling vs. Passive cycling) × Time (Baseline, 3-months, 6-months) mixed model analysis of variance (ANOVA). The role of physiological function as a mediator of the effect of FES cycling on mobility will be examined using bivariate correlation and multiple linear regression analyses. We hypothesize that there will be an improvement in walking performance and physiological function among individuals who receive FES cycling compared to those who receive passive cycling. We expect FES cycling will have a positive effect on walking performance through its influence on physiological function (i.e., cardiorespiratory fitness, muscular strength, and balance).
2.2. Participants
We plan to enroll a sample of 16 persons with MS to this trial. Participants will be recruited through advertisements in local media outlets. We will further distribute study information to individuals who have participated in previous research with our laboratory or who have inquired about previous exercise training studies, but were disqualified based on disability status (i.e., EDSS ≥ 6.0). The study will be described as an opportunity to participate in one of two leg cycling exercise programs. Participants will be asked to contact the Clinical Exercise Physiology Laboratory for information on study participation and screening for inclusion. The criteria for study inclusion are listed in Table 1. We will include participants irrespective of disease-modifying or symptomatic therapies, but will record this information at each testing session to control for potential covariates in data analysis.
Table 1.
Inclusion criteria for study participation.
| Criteria | Description |
|---|---|
| Age | 18–64 years of age |
| MS diagnosis | Confirmed diagnosis of MS based on Poser’s and/or McDonald’s criteria |
| Disability level | EDSS score = 6.0–6.5 (i.e., unilateral or bilateral assistance for ambulation) |
| Relapse-free | No history of a relapse within the past 30 days |
| Non-exerciser | Not currently participating in exercise on 2 or more days per week |
| Asymptomatic | No known cardiovascular, pulmonary, or metabolic disease or major signs or symptoms suggestive of these conditions based on the Physical Activity Readiness Questionnaire |
| Pregnancy | Not currently pregnant or plans to become pregnant during the study period |
| Contraindications to FES cycling | No known contraindications to FES cycling including epilepsy, a pacemaker, an implanted defibrillator, unstable fracture or implanted screws or pins |
| Physician approval | Physician approval for exercise testing and training |
NOTE: EDSS = Expanded Disability Status Scale; FES = functional electrical stimulation; MS = multiple sclerosis.
2.3. Sample size
We estimated the sample size for detecting a Condition [2 levels of between-subjects factor: FES cycling vs. Passive cycling] × Time [3 levels of within-subjects factor: 0, 3, and 6 months] interaction using the effect size [d = 0.73] from a published observational study of FES cycling on mobility in advanced MS [20] and assumptions of α = 0.05, β = 0.20, ICC = 0.50, and ε = 1.0. These parameters were selected based on the pilot nature of this investigation and the population that will be targeted. The minimal sample for testing the interaction should be 14 participants, and we will recruit 16 individuals with severe MS to account for potential attrition (∼10–15%).
2.4. Measures
2.4.1. Disability
A clinically-administered EDSS [23] examination will be conducted to confirm self-reported disability status and to describe the disability level of the sample. The EDSS will be performed by a member of the research team who is Neurostatus certified.
2.4.2. Timed 25-Foot Walk (T25FW)
The T25FW will be administered as a measure of walking speed, and will be administered according to standardized instructions [24]. Participants will be instructed to walk 25 feet as quickly as possible, but safely. An average of two walking trials in seconds will be computed and also converted to walking speed in m/s.
2.4.3. 2-Minute Walk (2MW)
The 2MW test will be administered as a measure of walking endurance and will be performed according to standardized instructions [25]. Participants will be asked to walk as fast and as far as possible for the duration of 2 min in an accessible corridor that is free of obstructions. A member of the research team will follow 1–3 m behind the participant with a measuring wheel to record the total distance traveled in meters. Based on the target disability level of the sample, the 2WM was selected as a measure of walking endurance to ensure that all participants will be able to complete the test.
2.4.4. Timed Up-and-Go (TUG)
The TUG test will be administered as a measure of walking agility [26], [27]. Participants will begin the task in a seated position with their back against the back of the chair. Participants will be asked to rise from the chair, walk 3 m, turn around, and then walk back to the chair and return to a seated position. Participants will complete two trials of the TUG test at a comfortable pace, and the time taken in seconds for each trial will be recorded.
2.4.5. Cardiorespiratory fitness (CRF)
CRF will be measured as peak oxygen consumption (VO2peak) using an incremental exercise test on a recumbent stepper (Nustep T5XR, Nustep Inc, Ann Arbor, MI). Expired gases will be collected using a two-way non-rebreathable valve and an open-circuit spirometry system (TrueOne, Parvo Medics, Sandy, UT) for analyzing expired gases [28], [29]. Participants will complete a 1-min warm-up at 15 W. The initial exercise intensity will be set to 15 W and will gradually increase by 5 W per minute until the participant can no longer continue exercising (i.e., volitional fatigue). Participants will wear a heart rate monitor (Polar Electro Oy, Finland) for continuous monitoring of heart rate response during exercise. Participants will be asked to rate their subjective experience of the exercise intensity using the Borg Rating of Perceived Exertion (RPE) scale. Heart rate and RPE will be recorded every minute during the test. VO2peak (ml/kg/min) will be determined as the highest recorded 20-s VO2 value when at least one of the following criteria are satisfied: (1) plateau in VO2 despite increases in work rate; (2) respiratory exchange ratio ≥ 1.10; (3) peak heart rate within 10 beats/minute of age-predicted maximum; or (4) peak rating of perceived exertion ≥ 17. This protocol has been developed and tested in individuals with severe MS disability (i.e., EDSS ≥ 6.0) [28].
2.4.6. Muscular strength
Bilateral isometric knee extensor and flexor peak torque will be measured using a Biodex System 3 dynamometer (Shirley, NY) [28]. Participants will be seated on the dynamometer according to the manufacturer’s recommendations with the hip and knee flexed at 90° and 60°, respectively. Participants will be asked to perform three, 5-s maximal knee extensions and three, 5-s maximal knee flexions. A 5-s rest period between each contraction attempt will be provided. Peak torque (Nm) will be determined as the highest recorded value for each muscle group.
2.4.7. Static balance
Static balance will be based upon assessments of the motion of the center of pressure (COP) quantified with a force platform (Bertec Corporation, Columbus, OH) [29]. Participants will stand without shoes on the force platform. Participants will not be permitted to use assistive devices, but will be permitted to wear ankle-foot orthoses as needed. We will collect two, 30-s trials on the force platform with a 30-s break between trials. Data collected from the trials will be quantified as the amount of postural motion indexed with sway area (based on a 95% confidence ellipse area) and velocity of postural sway along the anteroposterior (AP) and mediolateral (ML) axes [29].
2.5. Intervention
The FES cycling and passive cycling conditions will be delivered using RT300 cycles (see Fig. 2). The RT300 devices are FDA approved and consist of an electrically powered motor and multichannel FES controlled by a microprocessor and custom software. Both conditions will be delivered three days per week for the same duration, over 6 months. At each session we will record the distance traveled, pedaling energy in kcal, power in watts, and resistance in Nm, as well as heart rate and RPE. Each participant will be provided a log book for recording the training parameters as well as any adverse events experienced. Both interventions will be delivered by members of the research team trained in delivery of FES cycling and exercise training in persons with MS.
Fig. 2.
Photograph of the RT300 functional electrical stimulation cycles (Restorative Therapies Inc, Baltimore, MD).
2.5.1. FES cycling condition
Participants in the FES cycling condition will receive electrical stimulation during the exercise session. All participants will receive self-adhering surface electrodes (Pals Platinum, Axelgaard manufacturing CO, Ltd, Fallbrook, CA) that will be placed over three muscle groups of the lower extremities including the quadriceps, hamstrings, and gluteal groups. The microprocessor of the RT300 will generate an activation pattern of the leg muscles that results in a cycling motion. The FES stimulation parameters will be set as follows: waveform symmetric biphasic, phase duration 250 μs, and pulse rate 50 pulses per second. These parameters are consistent with existing FES cycling protocols in MS [20], [22]. The intensity and duration of leg cycling will be prescribed based on guidelines for aerobic exercise training developed for persons with MS [30] and from the American College of Sports Medicine (ACSM) [31]. Initially, the duration of exercise will be 10 min per session throughout the first month and will increase by 10 min each month until the participant is capable of performing 30 min of exercise. Exercise duration will be maintained at 30 min for months 4 through 6. Each session will begin and end with 5 min of passive leg cycling without electrical stimulation. Participants will be asked to maintain a cycling cadence of ∼40–50 rpm throughout the training sessions. The intensity of leg muscle stimulation will be adjusted per muscle group based on the patient’s sensory tolerance with the goal of maintain pedaling action and target heart rate over the entire session. Pedaling resistance will be adjusted in order to maintain exercise intensity.
2.5.2. Passive cycling condition
Patients in the passive cycling condition will undergo the same duration and frequency of training as participants in the FES cycling condition. However, participants will not receive any electrical stimulation. As a result, the pedaling motion will be generated entirely by the electric motor of the cycle ergometer. Cycling cadence will be maintained at ∼40–50 rpm. Participants will be reminded at the training sessions to refrain for actively participating in leg cycling. We will monitor heart rate and RPE during the training sessions as an indicator of participant effort. The passive cycling condition will control for attention and social contact associated with delivery of supervised FES cycling, and will allow us to understand the effect of FES cycling independent of changes that might occur in response to passive leg movement by the cycle ergometer.
2.6. Procedure
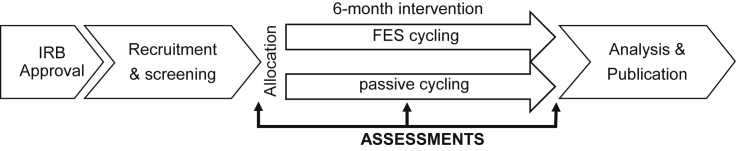
The study protocol will be approved by an Institutional Review Board. Fig. 1 provides a schematic of the study protocol. Interested participants will contact our laboratory for information on study participation. Participant screening will be conducted over the telephone and will involve the completion of a checklist based on inclusion criteria (see Table 1), the self-reported EDSS [32] and the Physical Activity Readiness Questionnaire (PAR-Q) [33]. This will allow for initial identification of persons with EDSS scores of 6.0–6.5 (i.e., unilateral or bilateral assistance for ambulation) who are inactive and asymptomatic based on the ACSM guidelines [31]. A letter describing the study components will be sent to the prospective participant’s physician(s) for obtaining approval for exercise testing and training, and for documenting the diagnosis of MS based on Poser’s and/or McDonald’s criteria [34], [35]. Once both letters are returned to the research team, we will schedule the baseline testing session for the provision of informed consent and assessment of neurological function, walking, and physiological function outcomes in the Exercise Neuroscience Research Laboratory. Participants will then be randomly assigned into either the FES cycling or passive cycling condition using a random numbers generator and concealed allocation. Participants will undertake the respective programs over a 6-month period administered through the Clinical Exercise Physiology Laboratory. Participants will undergo the same assessment protocol as with baseline in the middle and immediately after the 6-month period. Assessors will be blinded to condition allocation. Participants will receive $50 for completing the measurements on each of the three assessments.
Fig. 1.
Flow diagram of study protocol.
2.7. Statistical analyses
Data analysis will be performed using IBM SPSS Statistics 22.0 (IBM Corp., Armonk, IL). We will initially examine the data for outliers and non-normality. The effect of the intervention will be examined using Condition × Time mixed model ANOVA. Condition will be a between-subject factor and time will be a within-subject factor. Effect sizes associated with F-statistics will be expressed as eta-squared (η2). Effect sizes based on a difference in mean scores will be expressed as Cohen’s d. Bivariate correlations along with multiple linear regression will be used for examining physiological function variables as mediators of any FES cycling effect on mobility [36].
3. Discussion
This RCT will examine the efficacy of 6 months of supervised FES cycling for improving walking performance and physiological function among persons with severe MS disability. The results of this trial will provide important information for the design and delivery of a future RCT by: (1) providing effect sizes that can be included in a power analysis for optimal sample size estimation; and (2) identifying cardiorespiratory fitness, muscular strength, and balance (i.e., physiological function) as mechanisms for the beneficial effects of FES cycling on walking performance. FES cycling is a promising rehabilitation approach with the potential to significantly advance the management of progressive mobility disability using exercise training in severe MS.
There are several novel aspects of this study. First, we are examining exercise training among persons with severe MS disability, a cohort that has historically received minimal research attention regarding exercise training interventions [12]. The exercise stimulus itself (i.e., FES cycling) is novel, as there are few trials of FES cycling in persons with MS. To the best of our knowledge, there are five published trials (4 non-RCTs, 1 case study) that have examined FES cycling interventions in persons with MS [20], [21], [22], [37], [38]. These studies have demonstrated initial safety and feasibility, and preliminary benefits for walking performance, physical function, muscle strength and function, quality of life, and spasticity. However, these trials have primarily involved small samples, without a control condition, a limited number of training sessions, and a have not examined the mechanisms responsible for the benefits of FES cycling. These limitations are significant and necessitate a well-designed trial of FES cycling in severe MS. Our study will include a passive cycling condition that controls for social contact and leg movement by the ergometer. The intervention will involve 24 weeks (3 sessions/week) of FES cycling or passive cycling, and this is substantially longer than most previous interventions. We will examine physiological function outcomes (i.e., aerobic capacity, muscular strength, and balance) as mechanisms for the beneficial effects of FES cycling on walking performance. Overall, the design of this trial significantly improves upon previous research on FES cycling in MS and targets a specific patient group that is in need of alternative strategies for managing MS disability. This will provide important and much needed information on the potential benefits of FES cycling as an exercise rehabilitation tool.
If the proposed intervention is successful, the potential impact for MS patients, clinicians, and therapists is considerable. Importantly, disease modifying agents have limited efficacy in slowing the eventual progression of walking disability in severe MS [39], [40], [41]. Researchers have advocated for the development of powerful approaches that limit the progression of mobility disability [42], [43], [44], and rehabilitation has been recommended as the only practical means to reduce disability and restore function in MS [15]. Exercise-based rehabilitation using FES-cycling represents one potential approach for restoring and managing walking dysfunction in severe MS. If successful, FES cyling is an alternative exercise training modality that could be integrated within current rehabilitation regimens. There is tremendous long-term potential for FES cycling within the home and community environment. FES cycling is FDA approved for home-based therapy, the protocols can be self-administer, and exercise training data can be accessed remotely through the Internet for monitoring compliance and tracking progression. This highlights the ability of FES cycling programs to reach a substantial number of individuals with severe MS, and would address many of the barriers to exercise participation (e.g., lack of transportation) faced by this patient group.
We will conduct the first RCT of supervised FES cyling on outcomes of walking performance and physiological function compared to a credible control condition in patients with severe MS disability. This study will provide critical pilot data to design and implement a future large-scale RCT of FES cycling in persons with severe MS, and therefore represents a first step in this important line of research. Considering the limited options for therapeutic intervention, there is a critical need to explore innovative approaches for managing severe MS. FES cycling represents a novel exercise rehabilitation approach for managing disability, restoring function, and improving the lives of people with severe MS.
Acknowledgements
Supported, in part, by the National Multiple Sclerosis Society [PR-1411-02096] and the Consortium of Multiple Sclerosis Centers. The funding source played no role in the study design, writing of the report, and the decision to submit the article for publication.
References
- 1.Mayr W.T., Pittock S.J., McClelland R.L., Jorgensen N.W., Noseworthy J.H., Rodriguez M. Incidence and prevalence of multiple sclerosis in Olmsted County, Minnesota, 1985–2000. Neurology. 2003;61:1373–1377. doi: 10.1212/01.wnl.0000094316.90240.eb. [DOI] [PubMed] [Google Scholar]
- 2.Page W.F., Kurtzke J.F., Murphy F.M., Norman J.E., Jr. Epidemiology of multiple sclerosis in U.S. veterans: V. Ancestry and the risk of multiple sclerosis. Ann. Neurol. 1993;33:632–639. doi: 10.1002/ana.410330612. [DOI] [PubMed] [Google Scholar]
- 3.Hemmer B., Nessler S., Zhou D., Kieseier B., Hartung H.-P. Immunopathogenesis and immunotherapy of multiple sclerosis. Nat. Clin. Pract. Neurol. 2006;2:201–211. doi: 10.1038/ncpneuro0154. [DOI] [PubMed] [Google Scholar]
- 4.Trapp B.D., Nave K.-A. Multiple sclerosis: an immune or neurodegenerative disorder? Annu. Rev. Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 5.Bjartmar C., Trapp B.D. Axonal and neuronal degeneration in multiple sclerosis: mechanisms and functional consequences. Curr. Opin. Neurol. 2001;14:271–278. doi: 10.1097/00019052-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Pearson O., Busse M., van Deursen R., Wiles C. Quantification of walking mobility in neurological disorders. Quart. J. Med. 2004;97:467–475. doi: 10.1093/qjmed/hch084. [DOI] [PubMed] [Google Scholar]
- 7.Motl R.W. Physical activity and irreversible disability in multiple sclerosis. Exerc. Sport Sci. Rev. 2010;38:186–191. doi: 10.1097/JES.0b013e3181f44fab. [DOI] [PubMed] [Google Scholar]
- 8.Motl R.W., Goldman M.D., Benedict R.H. Walking impairment in patients with multiple sclerosis: exercise training as a treatment option. Neuropsychiatr. Dis. Treat. 2010;6:767–774. doi: 10.2147/NDT.S10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White L.J., Castellano V. Exercise and brain health-implications for multiple sclerosis: part 1-neuronal growth factors. Sports Med. 2008;38:91–100. doi: 10.2165/00007256-200838020-00001. [DOI] [PubMed] [Google Scholar]
- 10.Motl R.W., Pilutti L.A. The benefits of exercise training in multiple sclerosis. Nat. Rev. Neurol. 2012;8:487–497. doi: 10.1038/nrneurol.2012.136. [DOI] [PubMed] [Google Scholar]
- 11.Garrett M., Coote S. Multiple sclerosis and exercise in people with minimal gait impairment – a review. Phys. Ther. Rev. 2009;14:169–180. [Google Scholar]
- 12.Latimer-Cheung A.E., Pilutti L.A., Hicks A.L., Martin Ginis K.A., Fenuta A.M., MacKibbon K.A. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: a systematic review to inform guideline development. Arch. Phys. Med. Rehabil. 2013;94:1800–1828. doi: 10.1016/j.apmr.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 13.Snook E.M., Motl R.W. Effect of exercise training on walking mobility in multiple sclerosis: a meta-analysis. Neurorehabilit. Neural Repair. 2009;23:108–116. doi: 10.1177/1545968308320641. [DOI] [PubMed] [Google Scholar]
- 14.Coote S. Progressive resistance therapy is not the best way to rehabilitate deficits due to multiple sclerosis: yes. Mult. Scler. 2014;20:143–144. doi: 10.1177/1352458513515087. [DOI] [PubMed] [Google Scholar]
- 15.Kraft G.H. Rehabilitation still the only way to improve function in multiple sclerosis. Lancet. 1999;354:2016–2017. doi: 10.1016/S0140-6736(99)90035-1. [DOI] [PubMed] [Google Scholar]
- 16.Motl R.W. Ambulation and multiple sclerosis. Phys. Med. Rehabil. Clin. N. Am. 2013;24:325–336. doi: 10.1016/j.pmr.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Pilutti L.A., Hicks A.L. Rehabilitation of ambulatory limitations. Phys. Med. Rehabil. Clin. N. Am. 2013;24:277–290. doi: 10.1016/j.pmr.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Hunt K.J., Fang J., Saengsuwan J., Grob M., Laubacher M. On the efficiency of FES cycling: a framework and systematic review. Technol. Health Care. 2012;20:395–422. doi: 10.3233/THC-2012-0689. [DOI] [PubMed] [Google Scholar]
- 19.Deley G., Denuziller J., Babault N. Functional electrical stimulation: cardiorespiratory adaptations and applications for training in paraplegia. Sports Med. 2014;45:71–82. doi: 10.1007/s40279-014-0250-2. [DOI] [PubMed] [Google Scholar]
- 20.Ratchford J.N., Shore W., Hammond E.R., Rose J.G., Rifkin R., Nie P. A pilot study of functional electrical stimulation cycling in progressive multiple sclerosis. NeuroRehabilitation. 2010;27:121–128. doi: 10.3233/NRE-2010-0588. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds M.A., McCully K., Burdett B., Manella C., Hawkins L., Backus D. Pilot study: evaluation of the effect of functional electrical stimulation cycling on muscle metabolism in nonambulatory people with multiple sclerosis. Arch. Phys. Med. Rehabil. 2015;96:627–632. doi: 10.1016/j.apmr.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Fornusek C., Hoang P. Neuromuscular electrical stimulation cycling exercise for persons with advanced multiple sclerosis. J. Rehabil. Med. 2014;46:698–702. doi: 10.2340/16501977-1792. [DOI] [PubMed] [Google Scholar]
- 23.Kurtzke J.F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 24.Fischer J.S., Jak A.J., Knicker J.E., Rudick R.A., Cutter G.I. Demos Medical Publishing, Inc.; New York: 1999. Administration and Scoring Manual for the Multiple Sclerosis Functional Composite Measure (MSFC) [Google Scholar]
- 25.Goldman M.D., Marrie R.A., Cohen J.A. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult. Scler. 2008;14:383–390. doi: 10.1177/1352458507082607. [DOI] [PubMed] [Google Scholar]
- 26.Podsiadlo D., Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 27.Nilsagard Y., Lundholm C., Gunnarsson L.-G., Dcnison E. Clinical relevance using timed walk tests and “timed up and go” testing in persons with multiple sclerosis. Physiother. Res. Int. 2007;12:105–114. doi: 10.1002/pri.358. [DOI] [PubMed] [Google Scholar]
- 28.Pilutti L., Sandroff B., Klaren R., Learmonth Y.C., Platta M., Hubbard E. Physical fitness assessment across the disability spectrum in multiple sclerosis: a comparison of testing modalities. J. Neurol. Phys. Ther. 2015;39:241–249. doi: 10.1097/NPT.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 29.Sandroff B.M., Sosnoff J.J., Motl R.W. Physical fitness, walking performance, and gait in multiple sclerosis. J. Neurol. Sci. 2013;328:70–76. doi: 10.1016/j.jns.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 30.Latimer-Cheung A.E., Martin Ginis K.A., Hicks A.L., Motl R.W., Pilutti L.A., Duggan M. Development of evidence-informed physical activity guidelines for adults with multiple sclerosis. Arch. Phys. Med. Rehabil. 2013;94:1829–1836. doi: 10.1016/j.apmr.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 31.American College of SportsMedicine . Lippincott Williams & Wilkins; Philadelphia: 2013. ACSM’s Guidelines for Exercise Testing and Prescription. [Google Scholar]
- 32.Scheinberg L., Feldman F., Ratzker E. Self-assessment of neurological impairment in multiple sclerosis. Neurology. 1986;36:284. [Google Scholar]
- 33.Shephard R.J. PAR-Q, Canadian home fitness test and exercise screening alternatives. Sports Med. 1988;5:185. doi: 10.2165/00007256-198805030-00005. [DOI] [PubMed] [Google Scholar]
- 34.Poser C.M., Paty D.W., Scheinberg L., McDonald W.I., Davis F.A., Ebers G.C. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann. Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 35.Polman C.H., Reingold S.C., Banwell B., Clanet M., Cohen J.A., Filippi M. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann. Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baron R.M., Kenny D.A. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 37.Szecsi J., Schlick C., Schiller M., Pöllmann W., Koenig N., Straube A. Functional electrical stimulation-assisted cycling of patients with multiple sclerosis: biomechanical and functional outcome-a pilot study. J. Rehabil. Med. 2009;41:674–680. doi: 10.2340/16501977-0397. [DOI] [PubMed] [Google Scholar]
- 38.Krause P., Szecsi J., Straube A. FES cycling reduces spastic muscle tone in a patient with multiple sclerosis. NeuroRehabilitation. 2007;22:335–337. [PubMed] [Google Scholar]
- 39.Filippini G., Munari L., Incorvaia B., Ebers G.C., Polman C., D’Amico R. Interferons in relapsing remitting multiple sclerosis: a systematic review. Lancet. 2003;361:545–552. doi: 10.1016/S0140-6736(03)12512-3. [DOI] [PubMed] [Google Scholar]
- 40.Katrych O., Simone T.M., Azad S., Mousa S.A. Disease-modifying agents in the treatment of multiple sclerosis: a review of long-term outcomes. CNS Neurol. Disord. Drug Targets. 2009;8:512–519. doi: 10.2174/187152709789824598. [DOI] [PubMed] [Google Scholar]
- 41.Khan O., Leist T.P., Vollmer T.L., Zamvil S.S. Investigating multiple sclerosis: targeting therapeutic options. Int. J. MS Care. 2008;10:5–20. [Google Scholar]
- 42.Confavreux C., Vukusic S. The natural history of multiple sclerosis. Rev. Prat. 2006;56:1313–1320. [PubMed] [Google Scholar]
- 43.Confavreux C., Vukusic S., Moreau T., Adeleine P. Relapses and progression of disability in multiple sclerosis. N. Engl. J. Med. 2000;343:1430–1438. doi: 10.1056/NEJM200011163432001. [DOI] [PubMed] [Google Scholar]
- 44.Confavreux C., Vukusic S., Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain J. Neurol. 2003;126:770–782. doi: 10.1093/brain/awg081. [DOI] [PubMed] [Google Scholar]