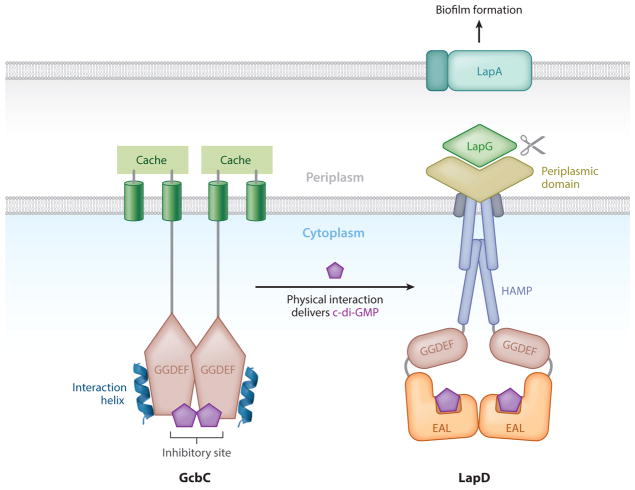
Figure 5.
GcbC physically delivers c-di-GMP to LapD. The inner membrane DGC requires physical contact to fully deliver its c-di-GMP signal to LapD. The interaction α5GGDEF helix of GcbC is depicted in blue. The inhibitory site of GcbC has also been shown to be a necessary element for interaction to occur and may help facilitate contact between these two proteins through an as yet undefined mechanism. c-di-GMP is depicted as purple pentagons located at the GcbC inhibitory site and bound at the LapD active site. Each protein functions as a dimer. GcbC has a cache domain that presumably allows enzymatic activity in response to an unidentified signal. The HAMP, GGDEF, EAL, and periplasmic domains of LapD are also shown. When LapD is activated by c-di-GMP, the periplasmic protease LapG is sequestered and the large adhesin LapA accumulates on the surface of the cell, allowing biofilm formation to commence. Adapted from Navarro et al. (45).