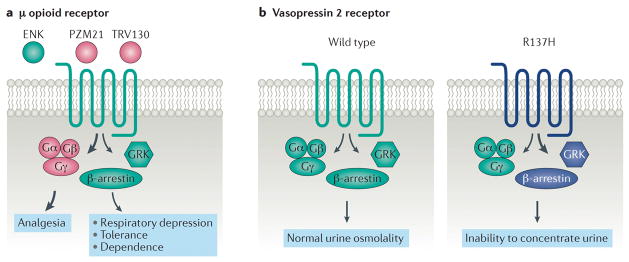
Figure 2. Drug discovery strategies and physiological consequences of biased signalling.
(A) The μ opioid receptor is a common drug target for analgesia. The endogenous ligand enkphalin (ENK) is unbiased (‘balanced’) with respect to G protein and βarrestin signaling. However, current μopioid selective agonists, such as morphine, that provide pain relief also cause adverse effects including respiratory depression, constipation, tolerance, and dependence. Both animal and human studies suggest that G protein signaling primarily mediates the analgesic efficacy, while βarrestin signaling mediates many of the adverse effects. Strongly G protein biased agonists, such as TRV130 and PZM21, may therefore provide clinical superiority to currently available agonists. (B) Relative to the wild-type receptor, the naturally occurring human vasopressin 2 receptor mutation R137H results in βarrestin biased signaling and is associated with familial nephrogenic diabetes insipidus. The vasopression R137H receptor variant is constitutively phosphorylated. This constitutive phosphorylation promotes greater βarrestin recruitment and internalization, even in the absence of arginine vasopressin (AVP, also known as antidiuretic hormone), compared to the wild-type receptor. Presumably, a significant reduction in vasopressin mediated Gαs signaling is sufficient to diminish aquaporin channel insertion into the renal collecting ducts, impeding urine concentration through reduced water resorption and leading to the clinical diagnosis of nephrogenic diabetes insipidus.