Table 2.
Ketone Variation for Cu-Catalyzed Enantioselective Reductive Couplings with Azadiene 1aa
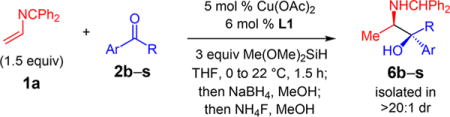
| ||||
|---|---|---|---|---|
|
| ||||
| entry | product, Ar, R | dr of 3b | yield (%)c | er of 6d |
| 1e | 6b, 4-MeOC6H4, Me | 3.5:1 | 60 | 96.5:3.5 |
| 2 | 6c, 4-F3CC6H4, Me | 4.0:1 | 50 | >99:1 |
| 3 | 6d, 4-N-pyrazolylC6H4, Me | 4.0:1 | 62 | >99:1 |
| 4e | 6e, 3-BrC6H4, Me | 3.5:1 | 45 | >99:1 |
| 5e | 6f, 3-ClC6H4, Me | 4.5:1 | 57 | >99:1 |
| 6 | 6g, 3-HOC6H4, Me | 7.5:1 | 62 | >99:1 |
| 7e | 6h, 2-BrC6H4, Me | >20:1 | 77 | 99:1 |
| 8 | 6i, 2-MeOC6H4, Me | 13.0:1 | 87 | 97:3 |
| 9 | 6j, 2-napthyl, Me | 3.5:1 | 65 | 99:1 |
| 10 | 6k, 3,4-dioxolatoC6H3, Me | 5.5:1 | 61 | 98.5:1.5 |
| 11 | 6l, 2-furyl, Me | 5.0:1 | 55 | >99:1 |
| 12 | 6m, 3-thiophenyl, Me | 9.0:1 | 83 | 99:1 |
| 13 | 6n, 3-pyrrolyl(NTs), Me | 5.5:1 | 58 | 95:5 |
| 14 | 6o, Ph, Et | 10.0:1 | 67 | 99:1 |
| 15 | 6p, Ph, CH2CH2Ph | 8.0:1 | 63 | 98:2 |
| 16 | 6q, 4-ClC6H4, 4-(i-PrO2CCMe2O)C6H4 | 1.0:1 | 39, 37f | 99:1, 99:1g |
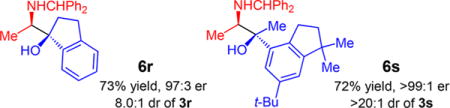
| ||||
Reaction with 0.2 mmol ketone 2.
Diastereomeric ratio of 3 determined by 400 MHz 1H NMR spectroscopy of the unpurified mixture prior to workup.
Isolated yield of purified 6 (>20:1 dr).
Enantiomeric ratio determined by HPLC analysis of 6.
Cu(OAc)2· H2O used.
Isolated yield of each diastereomer.
Enantiomeric ratio of each isomer.
