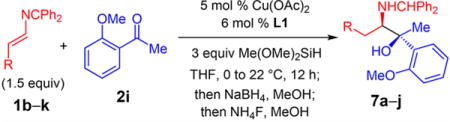
Table 3.
Substituted 2-Azadienes for Enantioselective Additions to Ketonesa
| ||||
|---|---|---|---|---|
|
| ||||
| entry | product, R | dr of 3b | yield (%)c | erd |
| 1 | 7a, n-Bu | >20:1 | 43 | 96.5:3.5 |
| 2e | 7b, (CH2)2Ph | >20:1 | 54 | 98.5:1.5 |
| 3 | 7c, (CH2)2(3-thiophenyl) | >20:1 | 52 | >99:1 |
| 4f | 7d, (CH2)2SMe | >20:1 | 52 | 98.5:1.5 |
| 5 | 7e, (CH2)3Ph | >20:1 | 45 | 98:2 |
| 6 | 7f, (CH2)3OBn | >20:1 | 47 | 98:2 |
| 7 | 7g, (CH2)3OPh | >20:1 | 59 | 99:1 |
| 8 | 7h, (CH2)3OTBS | >20:1 | 45 | 98.5:1.5 |
| 9 | 7i, (CH2)4OBz | >20:1 | 46 | 99:1 |
| 10 | 7j, (CH2)4C1 | >20:1 | 48 | 98.5:1.5 |
Reaction of (E)-azadiene 1 unless otherwise noted.
Determined by 400 MHz 1H NMR spectroscopy of the unpurified mixture prior to workup.
Isolated yield of purified 7.
Enantiomeric ratio determined by HPLC analysis of 7.
(E)- and (Z)-azadienes 1c deliver identical results.
From (Z)-1e.
