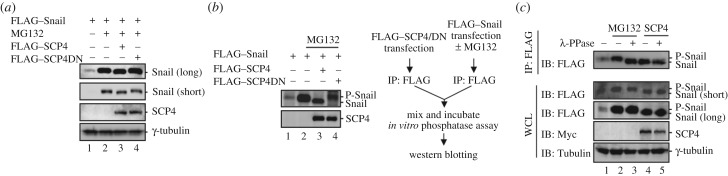
Figure 4.
SCP4 dephosphorylates Snail. (a) SCP4 causes Snail a migration shift in vivo. HEK293T cells were co-transfected with FLAG–Snail and FLAG–SCP4 or FLAG–SCP4DN. After 48 h, cells were treated with or without MG132 for 6 h. The protein level of Snail was detected by western blotting analysis. (b) SCP4 causes Snail a migration shift in vitro. In vitro reaction assay was carried out as described in the schema at the right. HEK293T cells were transfected with FLAG–Snail (with or without MG132 treatment) or FLAG–SCP4/DN to express respective proteins. Cell lysates were harvested by RIPA lysis buffer (150 mM NaCl, 20 mM Tris–HCl (pH 7.5), 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate). FLAG–Snail or FLAG–SCP4/DN proteins were purified by IP with anti-FLAG antibody, respectively. Purified FLAG–Snail and FLAG–SCP4 were incubated in in vitro phosphatase buffer at 30°C for 90 min. (c) SCP4 dephosphorylates Snail. HEK293T cells were co-transfected with FLAG–Snail and Myc-SCP4 or empty vector. After 48 h, cells were treated with or without MG132 for 6 h. Snail was immunoprecipitated from cell lysate and the immunocomplex was then incubated with or without λ-phosphatase for 30 min. Whole cell lysate and immunocomplex were subjected to western blotting analysis.