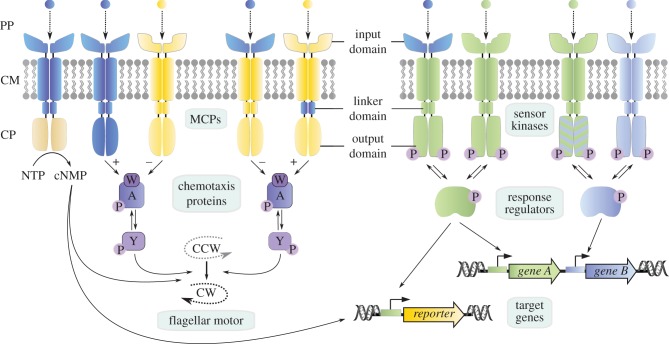
Figure 5.
Principles to rewire transmembrane signalling systems. Membrane-integrated methyl-accepting chemotaxis proteins (MCPs, left part) are generally composed of two modules: an input domain in the periplasm (PP) and in the cytoplasmic membrane (CM), which is responsible for ligand binding and signal transduction, and an output domain in the cytoplasm (CP), which induces a cellular response. Both domains are connected by a linker domain. Whereas input domains are highly diverse, variation in the output domains is rather limited. There are three common schemes for an output: nucleotide cyclase activity (NTP = nucleotide triphosphate → cNMP = cyclic nucleotide monophosphate) (outermost left); alterations of the direction of the flagellar motor (CCW = counter clockwise, CW = clockwise rotation) including the formation of a ternary complex between MCPs/CheW(W)/CheA(A) and (de-)phosphorylation of CheY(Y) (innermost left), and transcriptional regulation (right). Sensor kinases perceive a stimulus and transduce the signal via phosphorylation to a response regulator that acts as transcription factor of natural or reporter genes. The modular design of transmembrane signalling systems allows the generation of chimeric receptors in which the input, the linker or the output domain is replaced (domain colour switch). Sensor kinases can be rewired by amino acid replacement (blue/green stripes) to allow activation of a non-cognate response regulator.