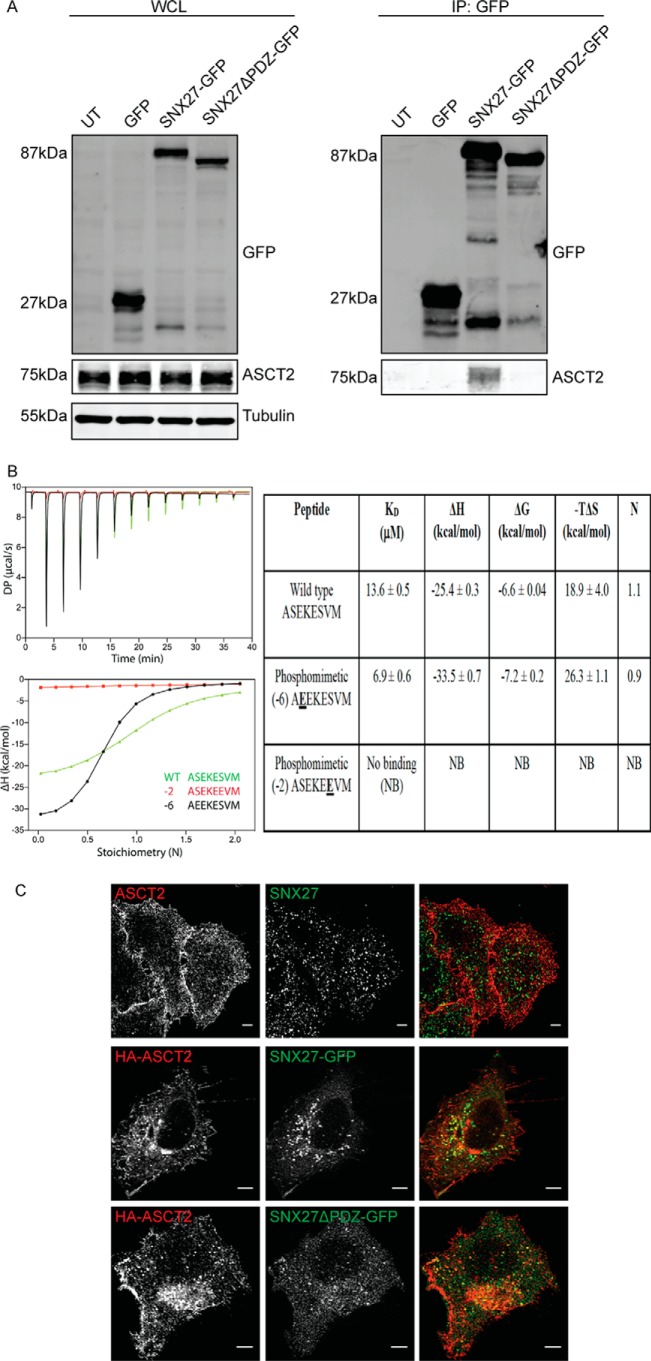
Figure 1.
SNX27 interacts directly with ASCT2. A, ectopically expressed GFP-SNX27 fusion proteins were immunoprecipitated (IP) by GFP-TRAP, and the binding of ASCT2 was determined by Western blotting as indicated. The calculated molecular weight for each protein is indicated. UT, untransfected; WCL, whole-cell lysate. B, the ASCT2 PDZbm peptide binds directly to the SNX27 PDZ domain in vitro. Top left panel, raw ITC data; bottom left panel, integrated normalized data and calculated Kd values. Peptides with phosphomimetic mutation at the −2 position are unable to bind SNX27, whereas phosphomimetic mutation at the −6 position enhances binding affinity and enthalpy. Binding parameters with SDs from three experiments are provided in the right panel. C, fixed and permeabilized HeLa cells were co-stained with endogenous ASCT2 and SNX27 antibodies. Alternatively, HA-ASCT2 was co-transfected with GFP-SNX27 constructs, as indicated, in HeLa cells. Indirect immunofluorescence was performed on fixed transfected cells to detect the HA epitope. Images were captured on a Zeiss LSM710 confocal microscopy using a ×60 objective. Scale bars = 5 μm.