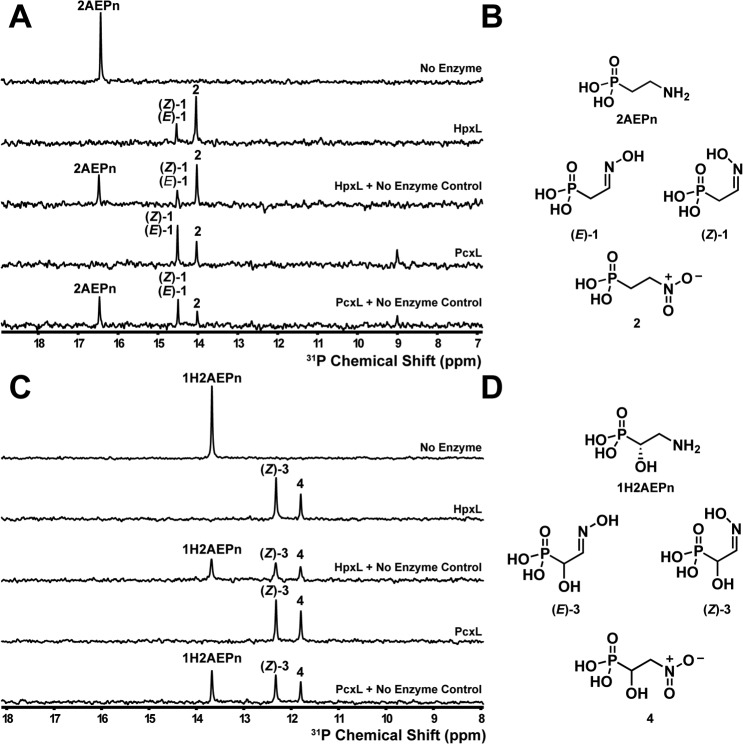
Figure 3.
Phosphorus NMR spectroscopy analysis of N-oxidase activity in the presence of 2AEPn or (S)-1H2AEPn. A, HpxL and PcxL form three major products in vitro when incubated for 16 h with NADPH, FAD, and 2AEPn as the substrate, whereas no product formation is observed in the absence of enzyme. B, these products have been assigned as the (E)- and (Z)-isomers of 2-iminoethylphosphonic acid (1) and 2-nitroethylphosphonic acid (2) based on the 1H-31P HMBC analysis (Figs. S2 and S3). Note that the (E)- and (Z)-isomers of 1 have identical 31P chemical shifts and thus display only a single peak in the one-dimensional 31P spectrum shown in A. C, HpxL and PcxL both generate two products in vitro using (S)-1H2AEPn as the substrate. D, these products were assigned as (Z)-1-hydroxy-2-hydroxyiminoethylphosphonic acid (3) and 1-hydroxy-2-nitroethylphosphonic acid (4) based on the 1H-31P HMBC analysis (Fig. S4 and S5). Chemical-mixing experiments with the control reaction were performed to confirm that substrate had been completely consumed. Substrates and cofactors were added at the following concentrations: 100 μm FAD, 500 μm NADPH, 3 mm 2AEPn or HpxV-generated (S)-1H2AEPn, 25 μm PcxL or HpxL, 25 mm phosphite, and 10 μm PTDH17x.