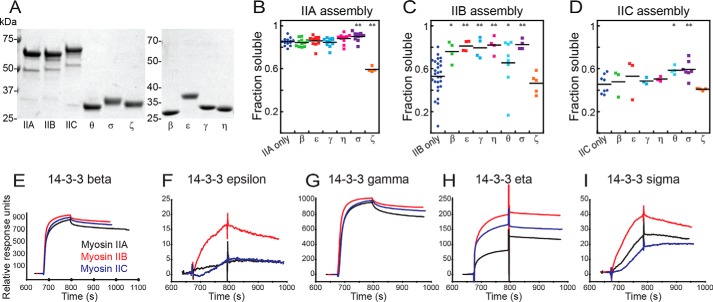
Figure 4.
The landscape of 14-3-3 interaction with human myosin IIs. A, images of Coomassie Brilliant Blue–stained SDS-PAGE gels of purified proteins used in this study. B–D, sedimentation assays of 1 μmdimer mCherry-IIA (B), mCherry-IIB (C), and mCherry-IIC (D), assembled at 150 mm NaCl, 37 °C, alone or in the presence of an equimolar 14-3-3 paralog (β, ϵ, γ, η, θ, σ, or ζ). Shown are post-ANOVA results of Fisher's LSD test versus negative control; *, p < 0.05; **, p < 0.005. E–I, surface plasmon resonance traces for each of the three immobilized, monomeric myosins bound by 14-3-3s: β (E), ϵ (F), γ (G), η (H), and σ (I).