Figure 5.
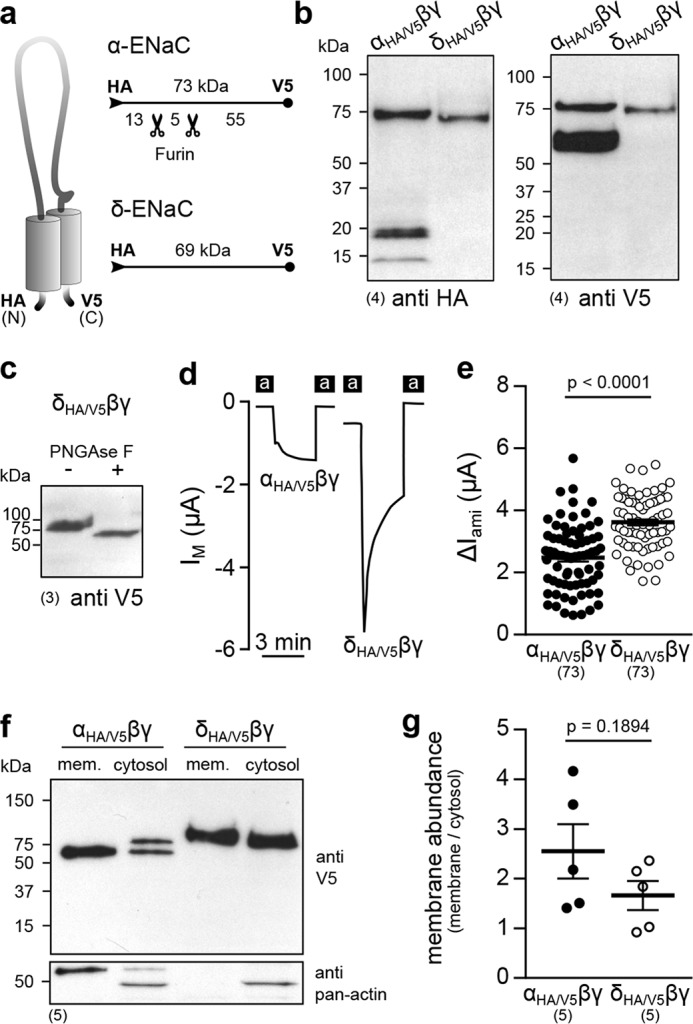
The δ-subunit is not subject to proteolytic maturation, and δβγ-ENaC membrane abundance is not changed compared with α-containing channels. a, schematic depiction of epitope-tagged ENaC subunits. The numbers indicate approximate molecular mass of peptides with/without furin cleavage, detected by immunoblots, as presented in b. b, immunoblots of whole-cell lysates from oocytes expressing αHA/V5βγ- or δHA/V5βγ-ENaC using anti-HA and anti-V5 antibodies. c, a migration shift of the δHA/V5-subunit due to treatment with PNGase F indicates glycosylation of the protein. d, representative IM recordings of oocytes expressing αHA/V5βγ- or δHA/V5βγ-ENaC. e, amiloride-sensitive current fractions (ΔIami) of αHA/V5βγ- and δHA/V5βγ-ENaC (Student's unpaired t test). f, immunoblot using an anti-V5-antibody of membrane (mem.) and cytosolic fractions from oocytes expressing αHA/V5βγ- or δHA/V5βγ-ENaC. The bottom blot shows the results from reprobing of the same membrane with an anti-pan-actin antibody to verify proper fractionation. g, membrane abundance of αHA/V5βγ- and δHA/V5βγ-ENaC derived from densitometric analysis of immunoblots as shown in f (Student's unpaired t test). Lines and error bars, mean and S.E.
