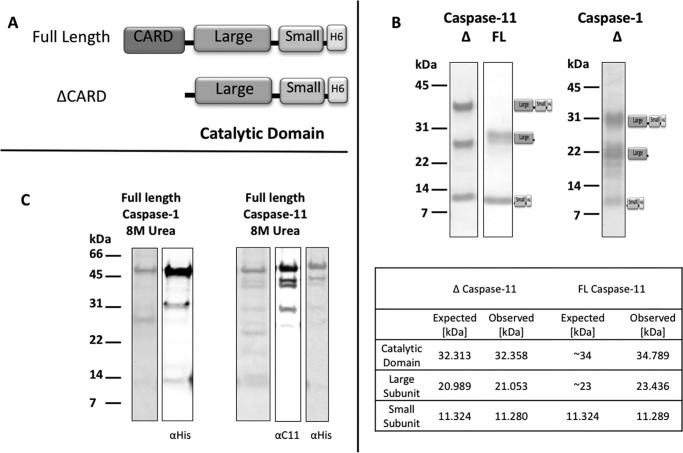
Figure 1.
Expression and purification of mouse caspase-1 and -11. A, two constructs, full-length (FL) and CARD-deleted (Δ), were designed for each enzyme, see “Materials and methods” for details of the constructs. B, purified ΔCARD caspase-1 and -11 resulted in a mixture of soluble, unprocessed catalytic subunit with processed large and small subunits. MALDI-MS analysis revealed molecular mass derivatives of ΔCARD caspase-11 that coincides with the expected engineered protein molecular weights. The CARD of FL caspase-11 was removed during expression to generate derivatives whose mass is consistent with processing at Glu97/Ser98. C, cell pellets expressing full-length enzymes were resuspended in 8 m urea, 50 mm Tris-Cl, 100 mm NaCl buffer, pH 8.0. Purified unfolded full-length enzymes were detected on 4–12% BisTris SDS-PAGE with Instant Blue and anti-His or caspase-11 antisera.