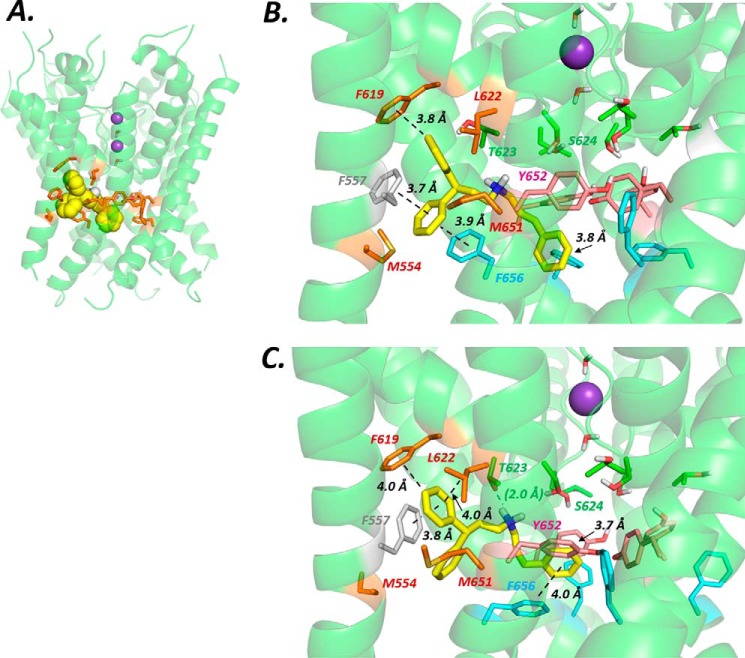
Figure 9.
A, location of one of four equivalent hydrophobic pockets in the pore domain of the hERG cryo-EM structure with Cavalli-2 docked in the configuration shown in B. Amino acid side chains that comprise the pocket (brown) were allowed to rotate freely during docking runs to accommodate the drug. Potassium ions (purple spheres) in the 1 and 3 positions of the selectivity filter and waters (in positions 2 and 4) were added for docking runs. Cavalli-2 is represented as a space-filling yellow surface. B, low-energy-score pose for Cavalli-2 docked into the hERG pore with docking biased to promote occupation of a hydrophobic pocket. In this run, rotamers of two Phe-656 side chains adjacent to the pocket containing Cavalli-2 were selected to orient the side chain Cα–Cβ bond toward the pore and fixed during docking to allow Cavalli-2 to interact with more than one Phe-656 side chain. Annotations define noncovalent interactions between drug and amino acid side chains according to the criteria in Table 2 of Dempsey et al. (29); only interactions that satisfy these criteria are annotated. C, as in B but no side chain rotamers were fixed during docking. In all structure figures, the hERG pore amino acid side chains are colored as follows: Phe-557, gray; Met-554, Phe-619, Leu-622, and Met-651, brown; Thr-623 and Ser-624, green; Tyr-652, pink; and Phe-656, blue. Cavalli-2 is yellow.