Table 1.
Aza-Friedel–Crafts Alkyl Imine Optimizationa
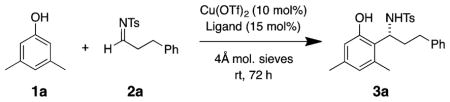
| |||||
|---|---|---|---|---|---|
| entry | solvent | ligand | phenol (equiv) | yield (%) | ee (%) |
| 1 | CH2Cl2 | (R)-Bn-Box | 5 | 19 | −43 |
| 2 | CH2Cl2 | (S)-i-Pr-Box | 5 | 23 | 69 |
| 3 | CH2Cl2 | (S)-t-Bu-Box | 5 | 5 | 37 |
| 4 | CH2Cl2 | (S)-Ph-Box | 5 | 54 | 84 |
| 5 | CH2Cl2 |
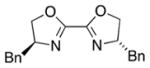
|
5 | 28 | <5 |
| 6 | CH2Cl2 |
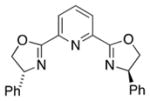
|
5 | 5 | −5 |
| 7 | CH2Cl2 | (4R,5S)-bis-Ph-Box | 5 | 7 | 91 |
| 8 | DCE | (S)-i-Pr-Quinox | 1.5 | 57 | <5 |
| 9 | CH2Cl2 | (S)-Ph-Box | 1.5 | 38 | 84 |
| 10 | xylenes | (S)-Ph-Box | 1.5 | 24 | 66 |
| 11 | THF | (S)-Ph-Box | 1.5 | 11 | 94 |
| 12 | toluene | (S)-Ph-Box | 1.5 | 16 | 75 |
| 13 | DCE | (S)-Ph-Box | 1.5 | 53 | 94 |
| 14 | PhCF3 | (S)-Ph-Box | 1.5 | 6 | 63 |
| 15 | EtOAc | (S)-Ph-Box | 1.5 | 36 | 94 |
| 16b | DCE | (S)-Ph-Box | 1.5 | 66 | 76 |
| 17c | DCE | (S)-Ph-Box | 1.5 | 49 | 92 |
| 18d | DCE | (S)-Ph-Box | 1.5 | 26 | 83 |
| 19e | DCE | (S)-Ph-Box | 1.5 | 39 | 87 |
| 20f | DCE | (S)-Ph-Box | 1.5 | 0 | — |
Reaction conditions: imine 2a (0.20 mmol) and phenol 1a (0.30 mmol) and flame-dried 4 Å mol. sieves were added to a solution of Cu(OTf)2 (0.020 mmol) and ligand (0.030 mmol) in 1 mL of solvent, and the mixture was stirred at rt for 72 h unless otherwise noted.
Reaction run at 40 °C.
Reaction performed at 0.3 M with respect to imine.
Reaction performed for 24 h.
Reaction performed for 48 h.
Reaction performed without Cu(OTf)2.
