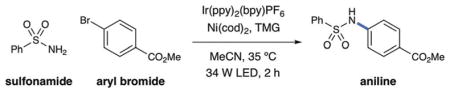
Table 1.
C–N sulfonamidation control experiments.[a]
| ||
|---|---|---|
| Entry | Conditions | Yield [%] |
| 1 | as shown | 99 |
| 2 | no light | 0 |
| 3 | no nickel | 0 |
| 4 | no photocatalyst | 11 |
| 5 | benzophenone (0.5%) as photocatalyst | 18 |
| 6 | no photocatalyst, no light | 0 |
| ||
Performed with Ir(ppy)2(bpy)PF6 (0.05 mol%), Ni(cod)2 (5 mol%), TMG (1.5 equiv), aryl halide (1.0 equiv), and benzenesulfonamide (1.5 equiv).
Yields were obtained by 1H NMR analysis.
