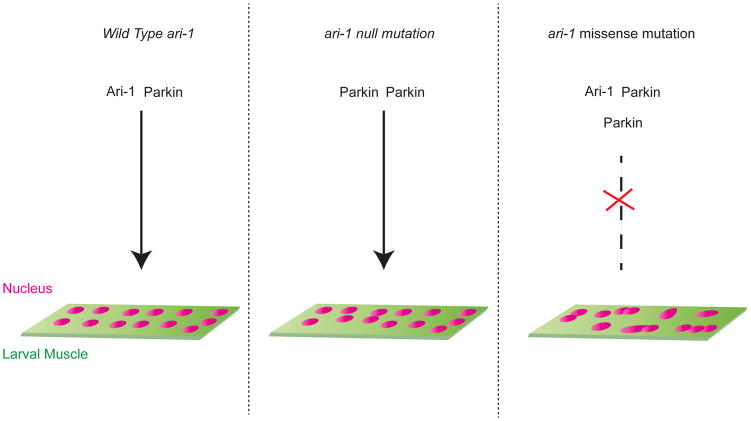
Figure 1. Ari-1 interacts with Parkin to facilitate myonuclear positioning.
(A) In a wildtype larval muscle myonuclear positioning is facilitated by the interaction of Ari-1 and Parkin, members of E3 ubiquitin ligase family, which regulate the turnover of a component of the LINC complex, Koi (Tan et al., Dev Cell) by ubquitinating Koi. (B) In an ari-1 null larval muscle, in the absence of Ari-1, Parkin forms a dimer and is able to partially regulate myonuclear positioning. (C) In larvae with a missense mutation in ari-1, mutant Ari-1 binds strongly to Parkin and perhaps to more than one Parkin, hindering the ability of both Ari-1 and Parkin to ubiquitinate Koi, which results in aberrant myonuclear positioning, nuclear clustering and defective nuclear morphology.