Abstract
Objective
Aneurysmal subarachnoid haemorrhage (SAH) may have devastating effects on patients. Motor and neurocognitive impairments may arise depending on the location and grade of the SAH. Although the effects of amantadine on neurocognitive function after traumatic brain injury have been widely studied to the best of our knowledge, their effects on recovery from SAH in humans have not been studied. The present study aimed to evaluate how amantadine influences improvement in neurocognitive function in patients with aneurysmal SAH over a period of six months.
Methods
This preliminary study included 12 patients with aneurysmal SAH who were admitted to the neurointensive care unit of Cerrahpasa Faculty of Medicine. Patients in Group A (n=5) received the standard treatment for SAH and amantadine for 30 days after admission, and those in Group C (n=7) received only the standard treatment. Neurocognitive function was evaluated using the Coma Recovery Scale-Revised and Disability Rating Scale on the first and fifth days and at the third and sixth months after admission. The primary endpoint of the present study was to compare the effects of amantadine in combination with the standard treatment to those of the standard treatment alone on the neurocognitive function of patients with SAH for over 6 months.
Results
Compared to the standard treatment alone, amantadine administration with the standard treatment during the early period of SAH may improve recovery.
Conclusion
Amantadine along with the standard treatment can ameliorate neurocognitive function after SAH.
Keywords: Amantadine, subarachnoid haemorrhage, neurocognitive functions
Introduction
Aneurysmal subarachnoid haemorrhage (SAH) is associated with high morbidity and mortality rates. Two main factors determining the morbidity and mortality rates due to SAH are re-bleeding and vasospasms. Therefore, the prevention of re-bleeding and management of vasospasms are essential in patient management (1). The main purpose of the current treatment is to maintain cerebral perfusion while preventing and controlling raised intracranial pressure (ICP) to evade secondary brain injury (2).
Subarachnoid haemorrhage causes excessive glutamate release associated with SAH-induced brain injury (3, 4). Glutamate release and glutamate N-methyl-D-aspartate (NMDA) receptor activation may be responsible for early and late ischemia as well as brain swelling in patients with SAH (4, 5).
Amantadine, a NMDA receptor antagonist, has been widely studied in patients with traumatic brain injury (TBI), and its efficacy on neurocognitive improvement after TBI has been demonstrated (6–10).
Memantine, which is a derivate of amantadine, is also a NMDA receptor antagonist, and its neuroprotective effects in SAH have been demonstrated in experimental animal studies (11–13). Memantine has also been found to have protective effects against SAH-induced vasospasms (14).
To the best of our knowledge, there is no human study that has evaluated the effects of the NMDA receptor antagonists on SAH-related neurocognitive damage.
Thus, the main aim of the present study was to evaluate the effects of amantadine on improvement in neurocognitive function in patients with aneurysmal SAH.
Methods
This prospective, randomised controlled study was performed between July 2014 and February 2017. After approval from the Ethics Committee of University of Istanbul, Cerrahpasa School of Medicine (Ethical Committee No: 83045809/604/01.01-114346; 4 July 2014) and written informed consent from the relatives of patients were received, 12 patients who were admitted to the neurointensive care unit with SAH due to cerebral artery aneurysm, who had Glasgow Coma Scale (GCS) scores of below 8/15 and who were aged between 18 and 70 years old were included in the study.
Patients presenting with heart failure, myocardial dysfunction, second- and third-degree atrioventricular blocks, bradycardia (<55 min−1), prolonged QT interval, arrhythmia, hypokalemia and hypomagnesemia were excluded from the study.
Patients were randomised to one of two groups using the sequentially numbered, opaque, sealed envelope technique. Amantadine was administered along with the standard SAH treatment protocol (Group A) in patients following neurointensive care unit admission, and only the standard treatment was administered to patients in the control group (Group C) (Figure 1).
Figure 1.
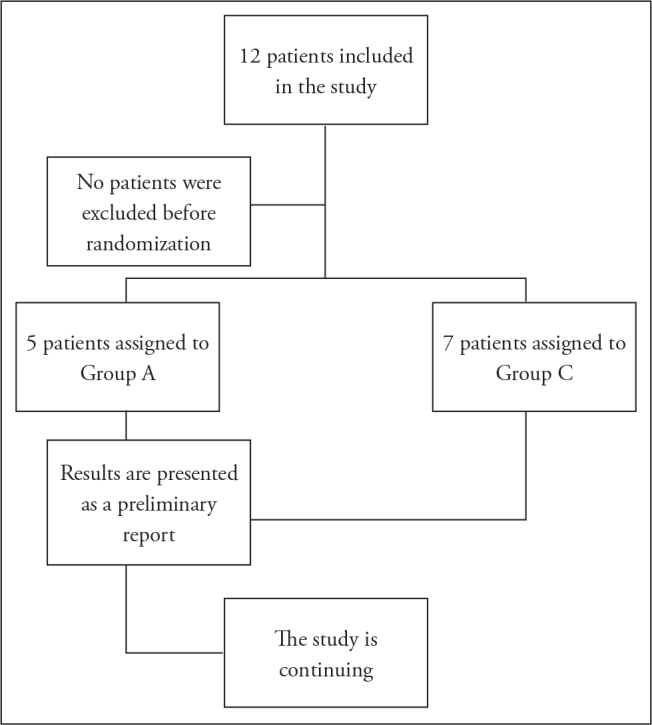
Study flowchart
The standard SAH treatment protocol included initial surgical clipping or endovascular coil embolisation of the ruptured aneurysm. Before aneurysm repair with surgical clipping or coil embolisation, the systolic arterial pressure was kept below 160 mmHg (1, 15). If the systolic arterial pressure was above 160 mmHg, intravenous (IV) esmolol infusion was administered (loading dose of 0.5 mg kg−1 over 1 min, then 0.05 mg kg min−1 for 4 min, in inadequate response in 5 min; 0.1 mg kg min−1 IV infusion).
Patients who had GCS scores below 8/15 on admission were mechanically ventilated in the volume-controlled mode with a tidal volume of 8 mL kg−1 (ideal body weight), inspiration: expiration ratio of 1:2, positive end-expiratory pressure (PEEP) of 5 cmH2O and respiratory rate (9–12 per min) adjusted to maintain PaCO2 in the range of 33–35 mmHg. After the acute phase, depending to the level of consciousness, extubation or tracheostomy was performed.
An external ventricular drainage (EVD) (Medtronic Neurosurgery®, USA) catheter was placed in patients who presented with hydrocephalus and/or who had Fisher grade IV SAH. Subsequently, the ICP and cerebral perfusion pressure (CPP) were monitored. Cerebrospinal fluid was drained via the EVD catheter to maintain the ICP below 20 mmHg. Osmotic diuresis with 20% mannitol or 3% sodium chloride was administered in case of an increased ICP despite the drainage, and in case of persistently increased ICPs, sedation was initiated (16). Between the 5th and 15th days after the initial SAH event, in the presence of a newly developed focal neurological deficit or decreased level of consciousness with or without fever, leukocytosis and hyponatremia were evaluated as symptoms of vasospasm. If vasospasm was suspected, digital subtraction angiography was performed to determine the diagnosis while euvolemia and hypertension were maintained. Noradrenalin infusion was administered to induce hypertension, and mean arterial pressures were adjusted to reach an individual CPP that improved the neurological deficit or level of consciousness. If the vasospasm continued despite euvolemia and hypertension, intra-arterial vasodilator use or angioplasty was planned.
Anticonvulsant prophylaxis with levetiracetam was administered to all patients and continued for 3–7 days. Long-term anticonvulsant therapy was continued in patients who had seizures.
Totally, 200 mg of IV amantadine was administered daily to Group A patients for five days after admission; then, 100 mg of amantadine was administered via the peroral/enteral route twice a day for 25 days.
The localisation of the ruptured aneurysm, the GCS score and the Fisher grades of the patients on admission and whether vasospasm or hydrocephalus developed were also recorded.
Neurocognitive function was recorded on admission to the neurointensive care unit (before amantadine administration for Group A; baseline), on the fifth day after admission and at the third and sixth months after admission date using the Coma Recovery Scale-Revised (CRS-R) (Table 1) and disability rating scale (DRS) (Table 2).
Table 1.
Coma recovery scale-revised
| Auditory Function Scale |
| 4- Consistent movement to command |
| 3- Reproducible movement to command |
| 2- Localization to sound |
| 1- Auditory startle |
| 0- None |
| Visual Function Scale |
| 5- Object recognition* |
| 4- Object Localization: Reaching* |
| 3- Visual pursuit* |
| 2- Fixation* |
| 1- Visual startle |
| 0- None |
| Motor Function Scale |
| 6- Functional object useβ |
| 5- Automatic motor response* |
| 4- Object manipulation* |
| 3- Localization to noxious stimulation* |
| 2- Flexion withdrawal |
| 1- Abnormal posturing |
| 0- None/Flaccid |
| Oromotor Function Scale |
| 3- Intelligible verbalization* |
| 2- Vocalization/Oral movement |
| 1- Oral reflexive movement |
| 0- None |
| Communication Scale |
| 2- Functional: Accurateβ |
| 1- Non-functional: Intentional* |
| 0- None |
| Arousal Scale |
| 3- Attention |
| 2- Eye opening with/without stimulation |
| 1- Unarousable |
| Total Score |
Denotes emergence from the minimally conscious state
Denotes minimally conscious state
Table 2.
Disability rating scale
| A. Eye Opening |
| 0- Spontaneous |
| 1- To speech |
| 2- To pain |
| 3- None |
| B. Communication Ability |
| 0- Oriented |
| 1- Confused |
| 2- Inappropriate |
| 3- Incomprehensible |
| 4- None |
| C. Motor Response |
| 0- Obeying |
| 1- Localizing |
| 2- Withdrawing |
| 3- Flexing |
| 4- Extending |
| 5- None |
| D. Feeding (Cognitive Ability Only) |
| 0. Complete |
| 1- Partial |
| 2- Minimal |
| 3- None |
| E. Toileting (Cognitive Ability Only) |
| 0. Complete |
| 1- Partial |
| 2- Minimal |
| 3- None |
| F. Grooming (Cognitive Ability Only) |
| 0. Complete |
| 1- Partial |
| 2- Minimal |
| 3- None |
| G. Level of Functioning (Physical, Mental, Emotional or Social Function) |
| 0- Completely independent |
| 1- Independent in social environment |
| 2- Mildly dependent-Limited assistance (non-resid – helper) |
| 3- Moderately dependent-Moderate assist (person in home) |
| 4- Markedly dependent-Assist all major activities, all times |
| 5- Totally dependent-24 hour nursing care |
| H. “Employability” (As a Full Time Worker, Homemaker or Student) |
| 0- Not restricted |
| 1- Selective jobs, competitive |
| 2- Sheltered workshop, non-competitive |
| 3- Not employable |
Total Score; 0 – None, 1 – Mild disability, 2 to 3 – Partial disability, 4 to 6 – Moderate disability, 7 to 11 – Moderately severe disability, 12 to 16 – Severe disability, 17 to 21 – Extremely severe disability, 22 to 24 – Vegetative state, 25 to 29 – Extreme vegetative state
The primary endpoint of the present study was to evaluate whether adding amantadine to the standard treatment improves neurocognitive function of patients with SAH over the initial 6 month period.
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS Inc.; Chicago, IL, USA) for Windows 15.0. All results are expressed as mean±SD and median.
Pearson’s chi-square test was used for the comparison of qualitative variables between the groups (such as gender, ASA physical status, localisation of the ruptured aneurysm, GCS scores and Fisher grades on admission, development of vasospasm and hydrocephalus) which showed binary change. The determination of normality and homogeneity of data distribution was performed using the Shapiro-Wilk test. The Mann-Whitney U test was used to compare non-normally distributed variables between the groups. The Wilcoxon signedrank test was used to compare non-normally distributed variables within the groups. Differences between the groups were analysed using independent samples t-test for age, body weight, height, CRS-R scores and DRS scores. Differences within the groups were analysed using the paired sample t-test. p≤0.05 were considered statistically significant.
Results
Twelve patients were included in this study. All patients survived during the six-month study period.
The patients in the study groups were similar in terms of gender, age, body weight, height and ASA physical status (p>0.05) (Table 3).
Table 3.
Patient characteristics
| Group A n=5 |
Group C n=7 | p | |
|---|---|---|---|
| Female gender n (%) | 2 (40%) | 3 (42.9%) | 0.92 |
| Age (years) (mean±SD) | 50.6±11.86 | 54.57±8.44 | 0.51 |
| Body weight (kg) (mean±SD) | 73±12.92 | 68.14±5.49 | 0.38 |
| Height (cm) (mean±SD) | 169.2±10.91 | 168.71±4.34 | 0.91 |
| ASA physical status (I/II/III) (n) | 3/2/0 | 3/4/0 | 0.46 |
The patients in the study groups were similar in terms of gender, age, body weight, height and ASA physical status (p>0.05). n: number of patients; ASA: American Society of Anesthesiologists
All patients underwent surgical clipping, had an EVD catheter paced and received anticonvulsant therapy. Two patients in Group A and three in Group C developed vasospasms. Three patients in Groups A and C developed hydrocephalus.
The patients in the study groups were similar in terms of the localisation of the ruptured aneurysms, GCS scores and Fisher grades on admission and the development of vasospasm and hydrocephalus (p=0.8, p=0.2, p=0.4, p=0.9 and p=0.9, respectively) (Table 4).
Table 4.
Localisation of the aneurysm, GCS scores and Fisher grades on admission, and frequency of development of vasospasm and hydrocephalus
| Group A n=5 |
Group C n=7 |
p | |
|---|---|---|---|
| Localisation of the aneurysm n (%) | 0.8 | ||
| MCA | 3 (60%) | 3 (42.8%) | |
| AComA | 2 (40%) | 2 (28.5%) | |
| ICA | 0 | 2 (28.5%) | |
| Basilar | 0 | 0 | |
| GCS score on admission n (%) | 0.3 | ||
| 3–4 | 0 | 0 | 0.2 |
| 5 | 1 (20%) | 2 (28.5%) | |
| 6 | 2 (40%) | 2 (28.5%) | |
| 7 | 2 (40%) | 3 (42.8) | |
| Fisher grades I/II/III/IV (n) | 0/0/3/2 | 0/0/4/3 | 0.4 |
| Vasospasm n (%) | 2 (40%) | 3 (42.8%) | 0.9 |
| Hydrocephalus n (%) | 3 (60%) | 3 (42.8%) | 0.9 |
The patients in the study groups were similar in terms of the localisation of the ruptured aneurysms, GCS and Fisher grades on admission, and frequency of the development of vasospasm and hydrocephalus (p>0.05). n: number of patients; MCA: Middle cerebral artery; AcomA: Anterior communicating artery; ICA: Internal carotid artery; GCS: Glasgow Coma Scale
The CRS-R scores on the fifth day and at the third and sixth months were significantly higher in Group A than in Group C (p=0.005, p=0.003 and p=0.003 respectively) (Figure 2).
Figure 2.
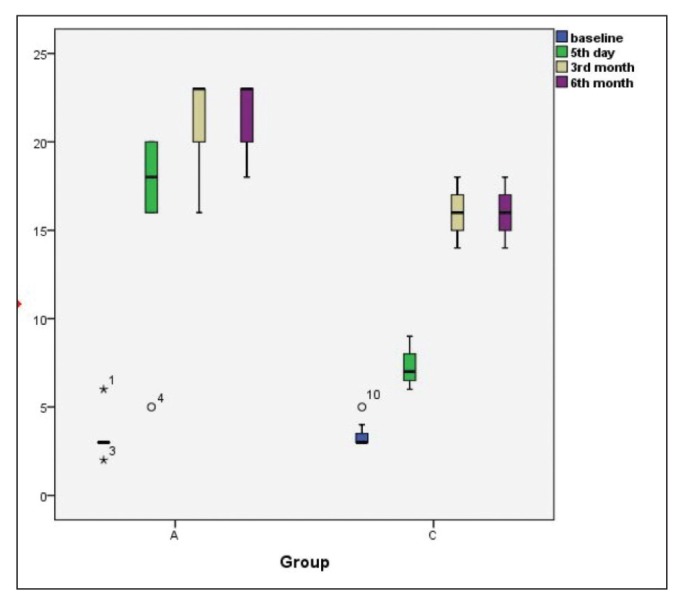
CRS-R scores at the indicated time intervals. Results are expressed as median (IQR). Outliner results were marked with symbols.
CRS-R: Coma Recovery Scale-Revised
The fifth-day and third- and sixth-month CRS-R scores were significantly higher than those at baseline in Group A (p=0.01, p=0.001, and p=0.04, respectively) and in Group C (p=0.017, p=0.001, and p=0.001, respectively) (Figure 2).
The DRS scores on the fifth day and at the third and sixth months were significantly lower in Group A than in Group C (p=0.031, p=0.001 and p=0.001, respectively) (Figure 3).
Figure 3.
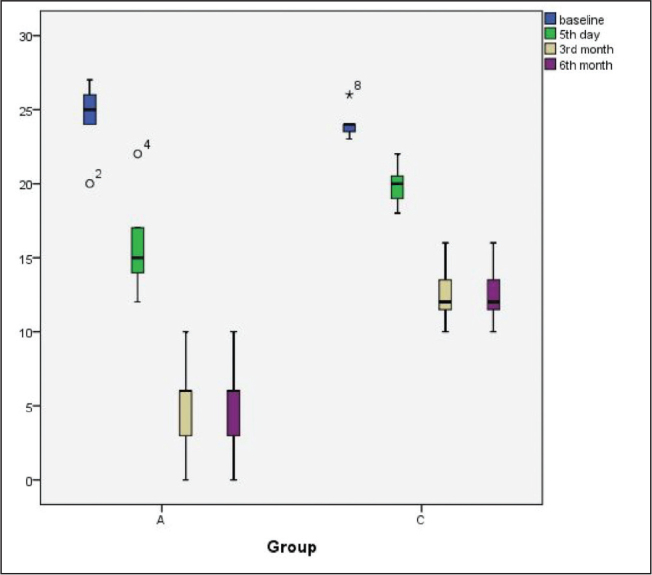
DRS scores at the indicated time intervals. Results are expressed as median (IQR). Outliner results were marked with symbols.
DRS: disability rating scale
The fifth-day and third- and sixth-month DRS scores were significantly lower than those at baseline in Group A (p=0.043) and Group C (p=0.018) (Figure 3).
Discussion
This study showed that the NMDA receptor antagonist amantadine administered with the standard treatment may better ameliorate neurocognitive function after aneurysmal SAH than the standard treatment only.
Approximately a quarter of patients with SAH die, and half of the survivors live with varying degrees of disability and neurocognitive impairment. Early repair of aneurysms and management of SAH-related complications, such as hydrocephalus and delayed cerebral ischemia, are thought to improve the functional outcome (15). Several studies on neuroprotection in SAH are being conducted (17). The drug memantine has been studied for this purpose, and its efficacy on neuroprotection in experimental SAH models has been demonstrated before (13). It was also found to be protective against SAH-induced vasospasm (14).
Glutamate is the major excitatory neurotransmitter in the brain. Excessive activation of NMDA-type glutamate receptors causes Ca2+ transport into the neurons, thereby causing neuronal excitotoxity (18). Memantine is an NMDA-type glutamate receptor antagonist, and this counteraction has been shown to be neuroprotective in many experimental studies (19, 20). Memantine is a derivative of amantadine (11). Both of them increase dopamine release in the central nervous system by affecting striatal neurons; furthermore NMDA-type glutamate receptor antagonism also enhances dopaminergic activity (21, 22). TBI is associated with reduction in the levels of catecholamines such as dopamine. The dopaminergic pathway is thought to regulate cognition and awareness (6, 8). Amantadine has been found to improve neurocognitive function and arousal after TBI due to its dopaminergic effect (7, 23).
Studies conducted with amantadine were conducted in patients with moderate-to-severe TBI (GCS=3–10/15) (7, 8). The patients in our study also had SAH at Fisher grade III–IV, and their GCS scores were below 8/15.
In the study conducted by Huang et al. (14), memantine was found to be protective against SAH-induced vasospasm in rats. The incidence of vasospasm was similar between the groups in our study.
Giacino et al. (7) compared amantadine with placebo in 184 patients with TBI and found that DRS scores indicated faster recovery rate with amantadine than with placebo. They administered amantadine 4–16 weeks after injury for four weeks. During the evaluation at six weeks, they concluded that recovery slowed after the discontinuation of amantadine. In our study, we initiated amantadine treatment on admission and continued treatment for a 30-day period. In the fifth-day and at the third-month evaluations, we found that adding amantadine for patient management better improved neurocognitive and behavioural function. Compared to the third-month evaluation, we did not observe any improvement or regression at the sixth-month evaluation. This difference might be due to our longer period of observation and the larger sample size in the other study.
Meythaler et al. (8) compared early administration (<6 weeks after TBI) to late administration (>6 weeks after TBI) of 200 mg of amantadine daily during 6 weeks in patients with TBI presenting with GCS scores below 10. They concluded that amantadine accelerates recovery, but they could not demonstrate the difference between the early and late initiation of amantadine administration. There is no consensus regarding the initiation time of amantadine administration in patients with TBI (6). Our patients were admitted to the neurointensive care unit in the first two days after SAH, and we initiated amantadine administration immediately after admission. We hypothesise that amantadine can block excitatory NMDA-type glutamate receptors during the acute period the injury, thus decreasing the severity of the insult.
Saniova et al. (24) compared standard therapy (mechanical ventilation with normocapnia, head position, sedation, temperature control, seizure control, euvolemia with CPP above 70 mm Hg, ICP below 20 mmHg, SjvO2 at 60–80%, and parenteral and enteral feeding) and amantadine to standard therapy alone in patients with TBI. They found that GCS scores were higher and mortality rates were lower in the amantadine group. In our study, we used the CRS-R and DRS scores to assess neurocognitive and behavioural status. The CRS-R scale give a summary of six measures including auditory, visual, motor, oromotor functions as well as communication and arousal (25). The DRS score evaluates the level of arousal, cognitive abilities, physical dependence on others and employability (26). These scoring systems are recommended for patients with disorders of consciousness because the distinction between the minimally conscious state and vegetative state are well recognised, particularly with the CRS-R (25, 26).
To the best of our knowledge, this study is the first that evaluated the effects of amantadine on neurocognitive recovery after SAH in humans. The results were impressive; therefore, we had a desire to share them as a preliminary report. For these reasons, the present study has a small sample size and is underpowered.
Conclusion
Amantadine can ameliorate neurocognitive function in patients with SAH. More studies with larger sample sizes should be conducted.
Footnotes
This study is presented in 51. Turkish Anesthesiology and Reanimation Congress in the form of oral presentation (25–29 October 2017 Antalya/Turkey).
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of İstanbul University Cerrahpaşa School of Medicine (No: 83045809/604/01.01-114346; 4 July 2014).
Informed Consent: Written informed consent was obtained from relatives of patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - Y.T.; Design - Ö.K.D., Y.T.; Supervision - Ö.K.D., Y.T.; Resources - E.F.A.; Materials - E.F.A., Ö.K.D, Y.T.; Data Collection and/or Processing - E.F.A., Ö.K.D., Y.T.; Analysis and/or Interpretation - E.F.A., Ö.K.D., H.V., Y.T.; Literature Search - E.F.A.; Writing Manuscript - E.F.A., Ö.K.D., H.V., Y.T.; Critical Review - Ö.K.D., Y.T.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Diringer MN, Bleck TP, Claude Hemphill J, 3rd, Menon D, Shutter L, Vespa P, et al. Neurocritical Care Society. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care. 2011;15:211–40. doi: 10.1007/s12028-011-9605-9. https://doi.org/10.1007/s12028-011-9605-9. [DOI] [PubMed] [Google Scholar]
- 2.Krivonos OV, Amosova NA, Smolentseva IG. Use of the glutamate NMDA receptor antagonist PK-Merz in acute stroke. Neurosci Behav Physiol. 2010;40:529–32. doi: 10.1007/s11055-010-9292-6. https://doi.org/10.1007/s11055-010-9292-6. [DOI] [PubMed] [Google Scholar]
- 3.Sarrafzadeh A, Haux D, Sakowitz O, Benndorf G, Herzog H, Kuechler I, et al. Acute focal neurological deficits in aneurysmal subarachnoid hemorrhage: relation of clinical course, CT findings, and metabolite abnormalities monitored with bedside microdialysis. Stroke. 2003;34:1382–8. doi: 10.1161/01.STR.0000074036.97859.02. https://doi.org/10.1161/01.STR.0000074036.97859.02. [DOI] [PubMed] [Google Scholar]
- 4.Persson L, Valtysson J, Enblad P, Warme PE, Cesarini K, Lewen A, et al. Neurochemical monitoring using intracerebral microdialysis in patients with subarachnoid hemorrhage. J Neurosurg. 1996;84:606–16. doi: 10.3171/jns.1996.84.4.0606. https://doi.org/10.3171/jns.1996.84.4.0606. [DOI] [PubMed] [Google Scholar]
- 5.de Lima Oliveira M, Kairalla AC, Fonoff ET, Martinez RC, Teixeira MJ, Bor-Seng-Shu E. Cerebral microdialysis in traumatic brain injury and subarachnoid hemorrhage: state of the art. Neurocrit Care. 2014;21:152–62. doi: 10.1007/s12028-013-9884-4. [DOI] [PubMed] [Google Scholar]
- 6.Sawyer E, Mauro LS, Ohlinger MJ. Amantadine enhancement of arousal and cognition after traumatic brain injury. Ann Pharmacother. 2008;42:247–52. doi: 10.1345/aph.1K284. https://doi.org/10.1345/aph.1K284. [DOI] [PubMed] [Google Scholar]
- 7.Giacino JT, Whyte J, Bagiella E, Kalmar K, Childs N, Khademi A, et al. Placebo-controlled trial of amantadine for severe traumatic brain injury. N Engl J Med. 2012;366:819–26. doi: 10.1056/NEJMoa1102609. https://doi.org/10.1056/NEJMoa1102609. [DOI] [PubMed] [Google Scholar]
- 8.Meythaler JM, Brunner RC, Johnson A, Novack TA. Amantadine to improve neurorecovery in traumatic brain injury-associated diffuse axonal injury: a pilot double-blind randomized trial. J Head Trauma Rehabil. 2002;17:300–13. doi: 10.1097/00001199-200208000-00004. https://doi.org/10.1097/00001199-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Spritzer SD, Kinney CL, Condie J, Wellik KE, Hoffman-Snyder CR, Wingerchuk DM, et al. Amantadine for patients with severe traumatic brain injury: a critically appraised topic. Neurologist. 2015;19:61–4. doi: 10.1097/NRL.0000000000000001. https://doi.org/10.1097/NRL.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 10.Zafonte RD, Watanabe T, Mann NR. Amantadine: a potential treatment for the minimally conscious state. Brain Inj. 1998;12:617–21. doi: 10.1080/026990598122386. https://doi.org/10.1080/026990598122386. [DOI] [PubMed] [Google Scholar]
- 11.Lipton SA. Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx. 2004;1:101–10. doi: 10.1602/neurorx.1.1.101. https://doi.org/10.1602/neurorx.1.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rammes G, Danysz W, Parsons CG. Pharmacodynamics of memantine: an update. Curr Neuropharmacol. 2008;6:55–78. doi: 10.2174/157015908783769671. https://doi.org/10.2174/157015908783769671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang CY, Wang LC, Wang HK, Pan CH, Cheng YY, Shan YS, et al. Memantine alleviates brain injury and neurobehavioral deficits after experimental subarachnoid hemorrhage. Mol Neurobiol. 2015;51:1038–52. doi: 10.1007/s12035-014-8767-9. https://doi.org/10.1007/s12035-014-8767-9. [DOI] [PubMed] [Google Scholar]
- 14.Huang CY, Wang LC, Shan YS, Pan CH, Tsai KJ. Memantine Attenuates Delayed Vasospasm after Experimental Subarachnoid Hemorrhage via Modulating Endothelial Nitric Oxide Synthase. Int J Mol Sci. 2015;16:14171–80. doi: 10.3390/ijms160614171. https://doi.org/10.3390/ijms160614171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connolly ES, Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43:1711–37. doi: 10.1161/STR.0b013e3182587839. https://doi.org/10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- 16.Dilmen OK, Akcil EF, Tunali Y. Intensive Care Treatment in Traumatic Brain Injury. Turk J Anaesthesiol Reanim. 2015;43:1–6. doi: 10.5152/TJAR.2014.26680. https://doi.org/10.5152/TJAR.2014.26680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James RF, Kramer DR, Aljuboori ZS, Parikh G, Adams SW, Eaton JC, et al. Novel Treatments in Neuroprotection for Aneurysmal Subarachnoid Hemorrhage. Curr Treat Options Neurol. 2016;18:38. doi: 10.1007/s11940-016-0421-6. https://doi.org/10.1007/s11940-016-0421-6. [DOI] [PubMed] [Google Scholar]
- 18.Urushitani M, Nakamizo T, Inoue R, Sawada H, Kihara T, Honda K, et al. N-methyl-D-aspartate receptor-mediated mitochondrial Ca(2+) overload in acute excitotoxic motor neuron death: a mechanism distinct from chronic neurotoxicity after Ca(2+) influx. J Neurosci Res. 2001;63:377–87. doi: 10.1002/1097-4547(20010301)63:5<377::AID-JNR1032>3.0.CO;2-#. https://doi.org/10.1002/1097-4547(20010301)63:5<377::AID-JNR1032>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist--a review of preclinical data. Neuropharmacology. 1999;38:735–67. doi: 10.1016/s0028-3908(99)00019-2. https://doi.org/10.1016/S0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- 20.Volbracht C, van Beek J, Zhu C, Blomgren K, Leist M. Neuroprotective properties of memantine in different in vitro and in vivo models of excitotoxicity. Eur J Neurosci. 2006;23:2611–22. doi: 10.1111/j.1460-9568.2006.04787.x. https://doi.org/10.1111/j.1460-9568.2006.04787.x. [DOI] [PubMed] [Google Scholar]
- 21.Wild AR, Akyol E, Brothwell SL, Kimkool P, Skepper JN, Gibb AJ, et al. Memantine block depends on agonist presentation at the NMDA receptor in substantia nigra pars compacta dopamine neurones. Neuropharmacology. 2013;73:138–46. doi: 10.1016/j.neuropharm.2013.05.013. https://doi.org/10.1016/j.neuropharm.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peeters M, Page G, Maloteaux JM, Hermans E. Hypersensitivity of dopamine transmission in the rat striatum after treatment with the NMDA receptor antagonist amantadine. Brain Res. 2002;949:32–41. doi: 10.1016/s0006-8993(02)02961-x. https://doi.org/10.1016/S0006-8993(02)02961-X. [DOI] [PubMed] [Google Scholar]
- 23.Kraus MF, Smith GS, Butters M, Donnell AJ, Dixon E, Yilong C, et al. Effects of the dopaminergic agent and NMDA receptor antagonist amantadine on cognitive function, cerebral glucose metabolism and D2 receptor availability in chronic traumatic brain injury: a study using positron emission tomography (PET) Brain Inj. 2005;19:471–9. doi: 10.1080/02699050400025059. https://doi.org/10.1080/02699050400025059. [DOI] [PubMed] [Google Scholar]
- 24.Saniova B, Drobny M, Kneslova L, Minarik M. The outcome of patients with severe head injuries treated with amantadine sulphate. J Neural Transm (Vienna) 2004;111:511–4. doi: 10.1007/s00702-004-0112-4. https://doi.org/10.1007/s00702-004-0112-4. [DOI] [PubMed] [Google Scholar]
- 25.Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85:2020–9. doi: 10.1016/j.apmr.2004.02.033. https://doi.org/10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 26.Rappaport M, Hall KM, Hopkins K, Belleza T, Cope DN. Disability rating scale for severe head trauma: coma to community. Arch Phys Med Rehabil. 1982;63:118–23. [PubMed] [Google Scholar]


