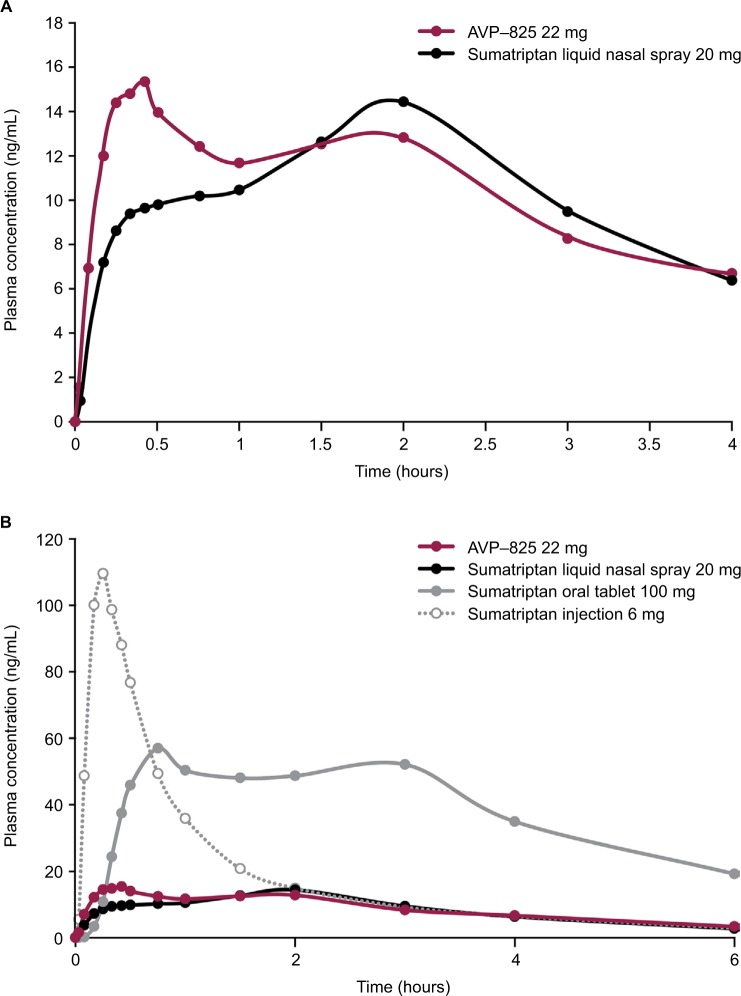
Figure 4.
Sumatriptan plasma concentration–time profiles over (A) the first 4 hours after AVP-825 (22 mg) and conventional sumatriptan liquid nasal spray (20 mg) and (B) the first 6 hours of the entire 14-hour sampling period for AVP-825, conventional sumatriptan liquid nasal spray (20 mg), sumatriptan oral tablet (100 mg), and subcutaneous sumatriptan injection (6 mg).
Note: Copyright ©2013. John Wiley and Sons Inc. Adapted from Obaidi M, Offman E, Messina J, Carothers J, Djupesland PG, Mahmoud RA. Improved pharmacokinetics of sumatriptan with Breath Powered™ nasal delivery of sumatriptan powder. Headache. 2013;53(8):1323–1333.29