Figure 2. Endpoint activity for KDACs with peptide substrates.
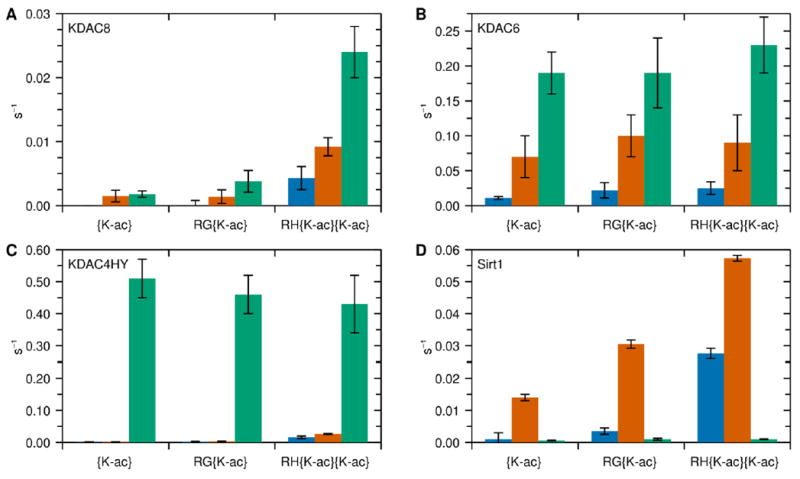
Three sets of peptide substrates where the C-terminus ends in an acetylated lysine (blue), tryptophan (orange), or AMC (green). Each peptide reacted with KDAC8 (A), KDAC6 (B), KDAC4HY (C), or Sirt1 (D) as described in the methods. The average specific activity and standard deviation for at least three replicates is shown for each enzyme-substrate pair. The differences in trends for each KDAC indicate that AMC conjugation has an effect on enzyme activity distinct from tryptophan, the amino acid most similar in structure.
