Figure 4. Sequence identification for products of doubly acetylated peptide reactions by tandem mass spectroscopy.
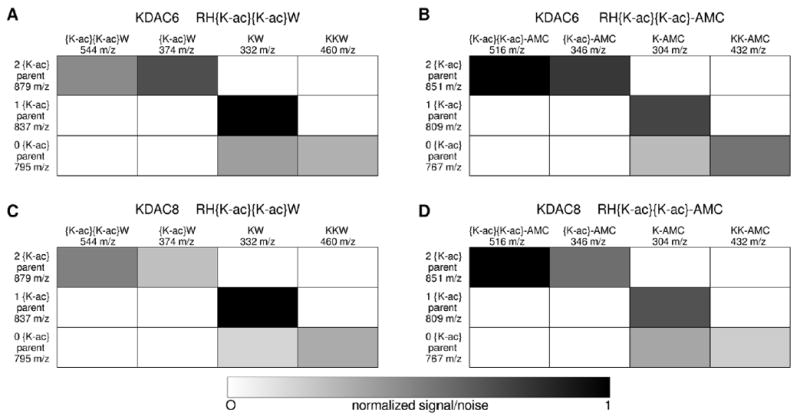
Reactions containing the highest concentration of enzyme from each of the pairs analyzed in Figure 3 were subjected to tandem mass spectrometry: KDAC6 with RH{K-ac}{K-ac}W (A), KDAC6 with RH{K-ac}{K-ac}-AMC (B), KDAC8 with RH{K-ac}{K-ac}W (C), and KDAC8 with RH{K-ac}{K-ac}-AMC (D). Substrate and product peptides (y-axis) were subjected to further fragmentation and the resulting y-ion fragments were analyzed. The signal/noise ratio for the diagnostic fragments (x-axis) are shown for each parent peptide, where white indicates a fragment that was not detected and black indicates high signal/noise ratios. All detected fragments were found to have at least 20% of the maximum signal/noise ratio, whereas all undetected fragments (white) were entirely absent. Diagnostic fragments are those that distinguish between deacetylation at lysine positions 3 and 4; other expected y-ion, a-ion, and b-ion peaks were observed but are not as useful for determining the acetylation pattern. Note that for the singly deacetylated peptide (1 {K-ac}) from each reaction pair, the diagnostic peptide fragment corresponding to deacetylation at position 4 (KW or K-AMC) was strongly detected, while the fragment corresponding to deacetylation at position 3 ({K-ac}W or {K-ac}-AMC) was always absent.
