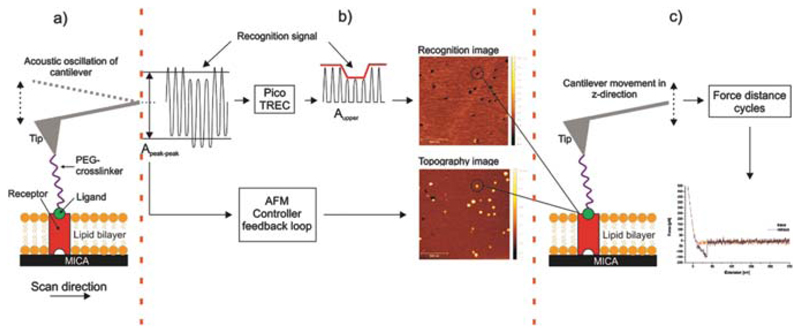
Fig. 2.
Experimental setup of combined TREC imaging and force spectroscopy. (a) Receptors (UCPs) reconstituted into a lipid bilayer formed on a mica surface and a single ligand (ATP) conjugated on a cantilever tip via a PEG crosslinker. (b) TREC mode: The cantilevers oscillation signal is split into two parts. (i) Aupper, the upwards oscillation peaks yields a map of recognition sites which are shown as dark dots in the recognition image. Molecules which did not bind to the ligand on the tip are invisible in the recognition image. (ii) Apeak–peak, downwards amplitude peaks lead to the topography image. (c) Switching into the force spectroscopy mode: repeatedly approaching and withdrawing of the ligand-carrying AFM tip on the previously selected receptor molecule to measure the interaction forces between these two molecules. Force versus distance data of force distance cycles, shown in red for the approaching period (trace) and in black for the retraction (retrace). In the latter a typical receptor-ligand unbinding event is visible as parabolic shaped downwards signal.