Abstract
Patients with T1 hepatocellular carcinoma (HCC) are not eligible for Model for End Stage Liver Disease (MELD) exception for liver transplant (LT) in part due to a high rate of misdiagnosis (no HCC on explant). The likelihood of misdiagnosis for T2 HCC and factors associated with misdiagnosis are unknown. We analyzed the Organ Procurement and Transplantation Network database including 5664 adults who underwent LT from 2012 to 2015 with MELD exception for T2 HCC, and searched for no evidence of HCC in the explant pathology file. We focused on those (n = 324) receiving no local-regional therapy (LRT) to evaluate the probability of no HCC found in explant. Median waiting time was short at 1.7 months, and 35 (11%) had no HCC on explant. On multivariable logistic regression, factors associated with no HCC on explant were age <50 (OR: 17.3, P < .001), non-HCV (OR: 5.4, P = .001), and alpha-fetoprotein <10 (OR: 2.9, P = .04). Tumor size and number were not different between groups. The proportion of misdiagnosis did not change significantly after implementation of Liver Imaging Reporting and Data System (LI-RADS) for HCC diagnosis. Conclusion: The rate of misdiagnosis was 11% among T2 HCC patients who underwent LT without receiving LRT prior to LT and did not change significantly after implementation of LI-RADS. More efforts are needed to eliminate unnecessary LT for patients without HCC.
Keywords: alpha-fetoprotein, Liver Imaging Reporting and Data System, Organ Procurement and Transplantation Network, United Network for Organ Sharing
1 | INTRODUCTION
The incidence of hepatocellular carcinoma (HCC) in the United States has tripled in the last 2 decades,1 and the demand for liver transplantation (LT) as a curative treatment option for HCC has continued to grow. HCC now accounts for more than 20% of all LT performed in the United States.2 In the Organ Procurement and Transplantation Network (OPTN) staging classification for HCC, the Milan criteria3 are further divided into stage T1 (1 lesion ≤1.9 cm) and T2 (1 lesion 2–5 cm, 2–3 lesions ≤3 cm). Since 2004, only patients meeting T2 criteria and not T1 have been granted priority listing status for LT, in large part due to a 30% rate of HCC misdiagnosis with no tumors found in the explant among those with presumed T1 HCC prior to LT.4 Furthermore, patients with T1 HCC are generally at low risk of waitlist dropout as a result of tumor progression to beyond T2 criteria.5,6
As more patients with HCC are competing with non-HCC patients for a scarce resource, standardized radiographic criteria are needed to ensure accurate diagnosis of HCC prior to LT. A typical HCC exhibits arterial phase enhancement and delayed phase wash-out on multiphase abdominal cross-sectional imaging, and they represent the key non-invasive diagnostic criteria endorsed by major liver societies.7,8 The Liver Imaging Reporting and Data System (LI-RADS) was launched in March 2011 to improve standardization and consensus regarding performance, interpretation, and reporting computed tomography or magnetic resonance imaging of the liver in patients with cirrhosis or other risk factors.9 The implementation of LI-RADS in October 2013 to determine priority listing for LT was intended to minimize the rate of HCC misdiagnosis.10 The impact of the LI-RADS system for HCC diagnosis among patients undergoing LT has not been fully elucidated.
In 2012, the OPTN online explant pathology form became available to capture tumor characteristics in the explant among patients undergoing LT for HCC. This provides the opportunity for assessment of the accuracy of pre-transplant HCC diagnosis. In this study, we aimed to determine the likelihood of misdiagnosis in patients presumed to have T2 HCC receiving priority listing for LT. We focused on those who did not receive local-regional therapy (LRT) before LT, as patients receiving LRT might have no tumor in the explant due to either complete response to treatment or misdiagnosis. We also sought to evaluate the impact of LI-RADS system on the rate of misdiagnosis, as well as factors associated with HCC misdiagnosis.
2 | PATIENTS AND METHODS
2.1 | Study design and patient population
This study included consecutive patients in the OPTN database aged 18 years and older who received MELD exception and underwent LT for T2 HCC between April 2012 and December 2015. This study start date was chosen as it is when the OPTN online explant pathology form became available. “No evidence of HCC” was a variable provided in the OPTN explant pathology file.1 Patients were excluded from this analysis if they received LRT prior to LT as it could not be determined if lack of HCC on explant was due to misdiagnosis or complete pathologic response to LRT. Patients were also excluded if their tumor burden ever exceeded Milan criteria on any exception petition.
In addition to presence or absence on HCC on explant, variables collected included demographic data (age, gender, race/ethnicity), liver-related factors (etiology of liver disease, Child-Pugh and Model for End Stage Liver Disease [MELD score]), and listing tumor characteristics including tumor burden and alpha-fetoprotein (AFP). Wait time from initial listing with MELD exception to LT as well as both region and center at which LT was performed was also collected. Patients were also subclassified according to whether they received LT before or after the implementation of LI-RADS in October 2013 for determining listing priority for MELD exception.
2.2 | Outcomes and statistical analysis
The primary outcome of interest was rate of misdiagnosis of HCC (indicated by no HCC on explant). Secondary outcomes included probability of post-LT survival, and recurrence based on whether HCC was found on explant. Patient characteristics were summarized using medians and interquartile ranges (IQR) for continuous variables and proportions for categorical variables. Logistic regression models with odds ratios (OR) were used to assess predictors of “no HCC on explant” and reported with 95% confidence intervals (CI). Loess curves were used to visualize the functional form of continuous variables. For those variables not demonstrating a linear relationship with the outcome, multiple cutoffs informed by the loess plots were tested to categorize the variables. Potential cutoffs were evaluated using Akaike information criterion (AIC) with lower AIC values indicating better model fit.11,12 Predictors of “no HCC on explant” with a univariate P value <.1 and the lowest AIC (when multiple cutoffs were tested) were included in the multivariable analysis with the final model selected by backward elimination (P for removal >.05). The Kaplan-Meier method was used to estimate post-transplant survival and HCC recurrence. Patient follow-up time was measured from the date of LT to death (for survival analysis), HCC recurrence (for recurrence analysis), or last follow-up. The log-rank test compared survival and HCC recurrence estimates by the presence or absence of HCC on explant. Statistical analyzes were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) and Stata/IC 11.1 (StataCorp, College Station, TX, USA).
3 | RESULTS
3.1 | Baseline clinical and tumor characteristics
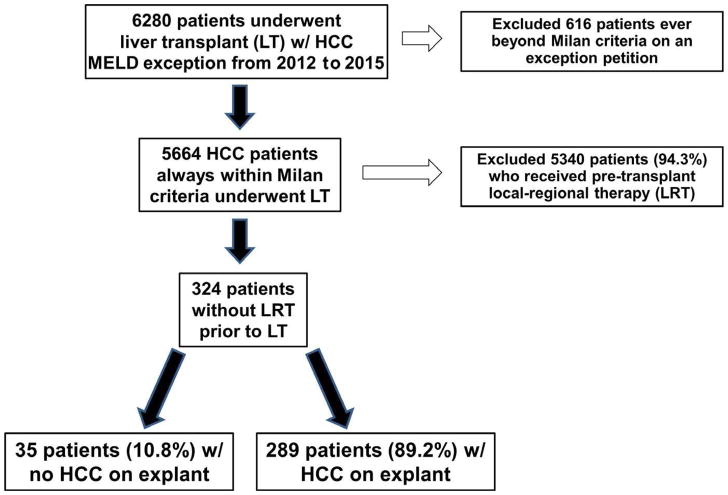
Among the 6280 patients in the OPTN database who were listed with MELD exception for HCC and received LT with a submitted explant pathology form, 616 patients (9.8%) had tumor burden exceeding Milan criteria on at least one exception petition and were excluded from the analysis. Of the remaining 5664 patients, 5340 (94.3%) received pre-transplant LRT. As lack of HCC on explant in these patients could have been due to complete response to LRT, these patients were also excluded. Of the 324 patients without LRT prior to LT, 35 (10.8%) lacked evidence of HCC on explant whereas HCC was present in the remaining 289 patients (89.2%; Figure 1).
FIGURE 1.
Flow diagram with exclusion criteria describing the formation of the study cohort with explant pathology demonstrating absence or presence of hepatocellular carcinoma
Baseline clinical characteristics of both the “no HCC on explant” group and “HCC on explant” group are shown in Table 1. Patients without HCC on explant were younger at listing with MELD exception (median age 48 vs 60 years, P < .001) and more often female (45.7 vs 26.0%, P = .02). In the “HCC on explant” group, hepatitis C was the most common etiology of liver disease (57.4%), followed by non-alcoholic fatty liver disease (13.8%), and alcoholic liver disease (8.0%). However, in the “no HCC on explant” group, hepatitis C was found in only 17.1% as was non-alcoholic fatty liver disease and autoimmune liver disease, while alcohol (25.7%) was the most common etiology of liver disease. At the time of initial listing with MELD exception, the median MELD and Child-Pugh score were similar amongst groups.
TABLE 1.
Baseline clinical characteristics of the “no hepatocellular carcinoma (HCC) on explant” group and “HCC on explant” group
| Overall (n = 324) | No HCC on Explant (n = 35) | HCC on explant (n = 289) | P-value | |
|---|---|---|---|---|
| Median age (IQR) | 60 (54–64) | 48 (35–61) | 60 (55–64) | <.001 |
|
| ||||
| Male (%) | 233 (71.9) | 19 (54.3) | 214 (74.0) | .02 |
|
| ||||
| Race/ethnicity (%) | ||||
|
| ||||
| Caucasian | 242 (74.7) | 25 (71.4) | 217 (75.1) | .15 |
| Hispanic | 38 (11.7) | 2 (5.7) | 36 (12.5) | |
| African American | 30 (9.3) | 7 (20) | 23 (8.0) | |
| Asian | 12 (3.7) | 1 (2.9) | 11 (3.8) | |
| Other | 2 (0.6) | 0 | 2 (0.7) | |
|
| ||||
| Etiology of liver disease (%) | ||||
|
| ||||
| Hepatitis C | 172 (53.1) | 6 (17.1) | 166 (57.4) | <.001 |
| NAFLD | 46 (14.2) | 6 (17.1) | 40 (13.8) | |
| Alcohol | 32 (9.9) | 9 (25.7) | 23 (8.0) | |
| Hepatitis B | 13 (4.0) | 3 (8.6) | 10 (3.5) | |
| Autoimmunea | 12 (3.7) | 6 (17.1) | 6 (2.1) | |
| Other | 49 (15.1) | 5 (14.3) | 44 (15.2) | |
|
| ||||
| Median Child’s score (IQR) | 9 (7–10) | 8 (7–9) | 9 (7–10) | .24 |
|
| ||||
| Median MELD (IQR) | 13 (10–16) | 14 (10–16) | 13 (10–16) | .54 |
|
| ||||
| Median AFP at listing (IQR) | 8 (4–24) | 4 (3–8) | 9 (4–28) | <.001 |
|
| ||||
| AFP <10 (%) | 177 (54.6) | 29 (82.9) | 148 (51.2) | .003 |
| AFP 10–100 (%) | 112 (34.6) | 5 (14.3) | 107 (37.0) | |
| AFP >100 (%) | 35 (10.8) | 1 (2.9) | 34 (11.8) | |
|
| ||||
| Tumor burden at listing (%) | ||||
|
| ||||
| 1 lesion 2–2.9 cm | 184 (56.8) | 20 (57.1) | 164 (56.7) | .90 |
| 1 lesion 3 cm–5 | 47 (14.5) | 4 (11.4) | 43 (14.9) | |
| 2 lesions | 74 (22.8) | 8 (22.9) | 66 (22.8) | |
| 3 lesions | 19 (5.9) | 3 (8.6) | 16 (5.5) | |
| Median wait time to LT (mo) | 1.7 (0.7–4.1) | 2.4 (0.8–4.6) | 1.5 (0.6–3.9) | .18 |
Includes autoimmune hepatitis, primary sclerosing cholangitis, and primary biliary cholangitis.
In terms of tumor characteristics at listing, patients without HCC on explant had a lower median AFP at listing (4 vs 9 ng/mL, P < .001). Median AFP was <10 ng/mL in 82.9% of those without HCC on explant vs 51.2% of those with HCC on explant (P = .003). There was no difference between groups in terms of listing tumor burden with median largest tumor diameter of 2.3 cm (IQR: 2.0–2.6) for “no HCC on explant” and 2.2 cm (2.0–2.6) for “HCC on explant” (P = .88). There were also no significant differences in the proportion of patients with a single lesion 2–3 cm or 2–3 lesions. Additionally, the proportion of patients with 2–3 lesions all <2 cm was similar between the two groups (22.9% vs 16.3%, P = .34). The median time from listing to LT was 1.7 months (IQR: 0.7–4.1) and did not differ between groups (2.4 months in no HCC on explant and 1.5 months with HCC on explant, P = .18; Table 1). Overall, the waiting time was <3 months in 65.1% and >6 months in 13.0%, with no significant differences between groups (P = .83).
Of the entire cohort, 58.3% underwent LT before the implementation of LI-RADS for determining eligibility for HCC MELD exception in October 2013. There was no difference found in the proportion of “no HCC on explant” found before and after the implementation of LI-RADS. No HCC on explant was found in 11.1% (21 of 189) of patients who underwent LT before October 2013 and 10.4% (14 of 135) of those who underwent LT after this date (P = .83).
3.2 | Geographic characteristics (region and LT center)
A breakdown of the overall cohort (n = 324) with respect to the 11 regions is shown in Table 2. The most common regions with patients without LRT prior to LT were Region 3 (29.0%), Region 11 (18.8%), and Region 10 (15.1%) and together these three regions accounted for 62.9% of the overall cohort. The least common regions with patients without LRT prior to LT were Region 1 (1.2%) and Region 9 (1.5%). When analyzing only those without HCC on explant (n = 35), Region 3 contributed the most number of patients (40.0%), which was more than the next three most common regions combined. No other region besides Region 3 (n = 14) had more than five patients without HCC on explant. There was not a single patient without HCC on explant in Regions 1, 6, and 9 (Table 2).
TABLE 2.
Breakdown of the “no hepatocellular carcinoma (HCC) on explant” and “HCC on explant” groups by United Network for Organ Sharing (UNOS) region and transplant center
| UNOS region | Total n = 324 (%) | No HCC on explant n = 35 (%) | HCC on explant n = 289 (%) |
|---|---|---|---|
| 1 | 4 (1.2) | 0 | 4 (1.4) |
| 2 | 32 (9.9) | 4 (11.4) (n at each center = 2, 1, 1) | 28 (9.7) |
| 3 | 94 (29.0) | 14 (40.0) (n = 3, 3, 2, 1, 1, 1, 1, 1, 1) | 80 (27.7) |
| 4 | 15 (4.6) | 1 (2.9) | 14 (4.8) |
| 5 | 17 (5.2) | 5 (14.3) (n = 2, 1, 1, 1) | 12 (4.2) |
| 6 | 11 (3.4) | 0 | 11 (3.8) |
| 7 | 17 (5.2) | 4 (11.4) (n = 3, 1) | 13 (4.5) |
| 8 | 19 (5.9) | 2 (5.7) (n = 1, 1) | 17 (5.9) |
| 9 | 5 (1.5) | 0 | 5 (1.7) |
| 10 | 49 (15.1) | 3 (8.6) (n = 2, 1) | 46 (15.9) |
| 11 | 61 (18.8) | 2 (5.7) (n = 1, 1) | 59 (20.4) |
With respect to LT center, the 35 patients without HCC on explant received LT at 25 different centers. The maximum number of LT recipients without HCC on explant at a single center was three which occurred at two centers in Region 3 and a single center in Region 7 (Table 2). The majority of centers (69.1%, n = 56/81) who performed at least one LT without pre-transplant LRT during the study time period did not have a single patient without HCC on explant.
3.3 | Predictors of “no HCC on explant”
The results of univariate and multivariable analysis of predictors of “no HCC on explant” are summarized in Table 3. Predictors of “no HCC on explant” in univariate logistic regression included female gender (OR: 2.40, 95% CI: 1.18–4.91, P = .02), African American race (OR: 2.64, 95% CI: 1.03–6.78, P = .04), and non-hepatitis C etiology of liver disease (OR: 6.52, 95% CI: 2.63–16.20, P < .001). Additionally, listing age at all tested cutoffs including <50, <55, and <60 years and AFP at cutoffs including <5, <10, and <20 ng/mL were significant predictors of no HCC on explant. Only 50.0% (19/38) of patients <50 years old who received LT for presumed HCC were actually found to have HCC on explant. There were no individual United Network for Organ Sharing (UNOS) regions which reached statistical significance for predicting “no HCC on explant,” although Region 3 (OR: 1.74, P = .13) and Region 11 (OR: 0.24, P = .052) nearly did. Child-Pugh, and MELD score, listing tumor burden, and wait time from listing to LT were not found to be predictive of “no HCC on explant” on univariate analysis.
TABLE 3.
Univariate and multivariable analyzes of predictors of “no hepatocellular carcinoma on explant”
| Predictor variables | Odds ratio (95% CI) | P-value |
|---|---|---|
| Univariate analysis | ||
| Age | ||
| <50 vs ≥50 | 16.88 (7.49–38.00) | <.001 |
| <55 vs ≥55 | 5.83 (2.78–12.21) | <.001 |
| <60 vs ≥60 | 2.66 (1.2–5.74) | .01 |
| Female gender | 2.40 (1.18–4.91) | .02 |
| African American (vs Caucasian) | 2.64 (1.03–6.78) | .04 |
| Non-hepatitis C etiology (vs hepatitis C) | 6.52 (2.63–16.20) | <.001 |
| Child-Pugh score (per point) | 0.90 (0.76–1.07) | .22 |
| MELD score (per point) | 1.02 (0.94–1.10) | .69 |
| AFP at listing | ||
| <5 vs ≥5 | 3.21 (1.57–6.56) | .001 |
| <10 vs ≥10 | 4.61 (1.86–11.43) | <.001 |
| <20 vs ≥20 | 7.10 (1.67–30.26) | .008 |
| Tumor burden at listing | ||
| 1 lesion 3–5 cm (vs 1 lesion 2–2.9 cm) | 0.77 (0.25–2.35) | .64 |
| 2 lesions (vs 1 lesion 2–2.9 cm) | 0.99 (0.42–2.37) | .99 |
| 3 lesions (vs 1 lesion 2–2.9 cm) | 1.54 (0.41–5.74) | .52 |
| Median total tumor diameter <3 vs ≥3 cm | 1.21 (0.56–2.61) | .64 |
| Wait time from listing to LT | ||
| >3 vs ≤3 mo | 1.28 (0.62–2.62) | .50 |
| >6 vs ≤6 mo | 1.14 (0.41–3.11) | .81 |
| >12 vs ≤12 mo | 1.69 (0.36–8.05) | .51 |
| UNOS regiona | ||
| Region 2 vs all other regions | 1.20 (0.40–3.66) | .75 |
| Region 3 vs all other regions | 1.74 (0.85–3.59) | .13 |
| Region 10 vs all other regions | 0.50 (0.15–1.69) | .26 |
| Region 11 vs all other regions | 0.24 (0.06–1.01) | .052 |
| Multivariate analysis | ||
| Age <50 vs ≥50 | 17.30 (6.99–42.83) | <.001 |
| Non-hepatitis C etiology (vs hepatitis C) | 5.43 (1.94–15.20) | .001 |
| AFP <10 vs ≥10 | 2.87 (1.02–8.06) | .04 |
Only analyzed individual regions who had at least 20 patients of the entire cohort.
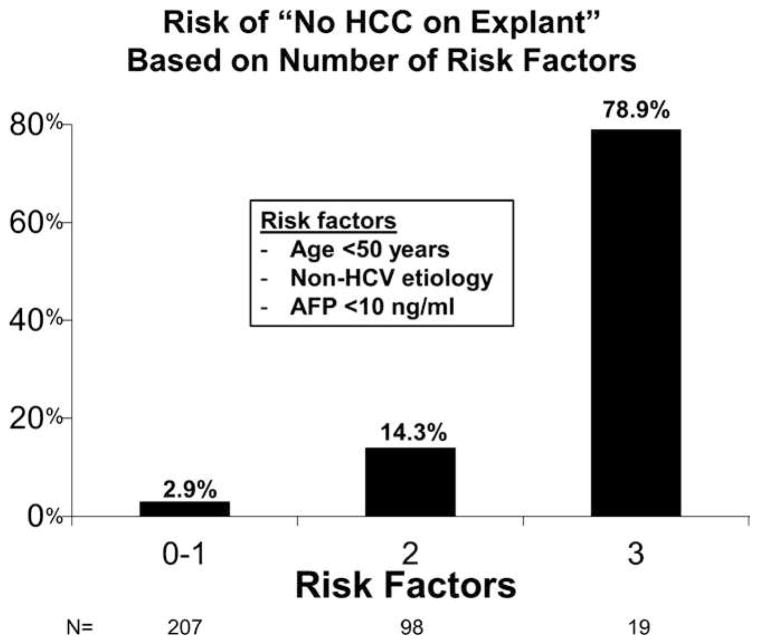
There were three predictors of “no HCC on explant” found on multivariable logistic regression: age <50 years (OR: 17.30, 95% CI: 6.99–42.83, P < .001), non-hepatitis C etiology (OR: 5.43, 95% CI: 1.94–15.20, P = .001), and AFP <10 ng/mL at LT listing (OR: 2.87, 95% CI: 1.02–8.06, P = .04). When patients were stratified by the number of risk factors found on multivariable analysis, 63.9% had 0–1 risk factors, 30.2% had 2 risk factors, and 5.9% had all three risk factors. No HCC on explant was observed in 2.9% (6/207) of those with 0–1 risk factors, 14.3% (14/98) with 2 risk factors, and 78.9% (15/19) of patients with all 3 risk factors (P < .001; Figure 2).
FIGURE 2.
Risk of “no hepatocellular carcinoma on explant” based on number of risk factors identified from results of multivariable analysis: age <50, non-HCV etiology of liver disease, and alpha-fetoprotein (AFP) <10
3.4 | Post-LT outcomes
Median post-LT follow-up time was 1.8 years (IQR 0.7–2.2). Overall Kaplan-Meier post-LT survival at 1 and 3 years was 92.1% (95% CI: 88.3–94.7) and 81.7% (73.8–87.4), respectively.
The observed 3-year post-LT survival was higher at 90.8% among those without HCC on explant when compared to the 3-year post-LT survival of 80.3% in those with HCC on explant, although the difference did not achieve statistical significance (P = .39). No post-LT HCC recurrence was identified among the 35 patients without HCC on explant, whereas the Kaplan-Meier probability of HCC recurrence 3 years after LT was 4.4% (95% CI: 1.9–9.8) in those with HCC found on explant (P = .28).
4 | DISCUSSION
Harper et al13 recently analyzed over 4500 LT recipients in the OPTN explant pathology file in place since April 2012 and found that 22.7% of patients exceeded T2 criteria on explant. The rate of under staging did not change significantly after LI-RADS implementation. In the present study, we focused on the rate of misdiagnosis in patients undergoing LT for presumed T2 HCC who did not receive LRT prior to LT, as the absence of viable HCC in the explant could be due to either misdiagnosis or a complete response to LRT. Our analysis on patients who did not receive any LRT prior to LT demonstrated an 11% rate of misdiagnosis with no HCC in the explant. The median waitlist time in these patients was very short at only 1.7 months. The rate of misdiagnosis did not change significantly after the implementation of LI-RADS in October 2013. This observation suggests that accurate interpretation of imaging using the LI-RADS system may still be a work in progress. It has been shown that a significant interob-server variability exists among radiologists to diagnose and stage HCC based on cross-sectional imaging.14 This also brings up the question of whether there are specific subgroups with T2 HCC that are more prone to diagnostic errors.
We have originally hypothesized a higher misdiagnosis rate in a subset of T2 HCC with multiple small lesions all under 2 cm. MRI is only 52–62% sensitive for the diagnosis of small tumors <2 cm,15,16 and smaller tumors appear to show less pronounced delayed washout compared to larger HCC.17 Samoylova et al18 previously found that post-LT HCC recurrence risk was significantly lower in patients with 2–3 nodules all <2 cm compared to others within stage T2, raising the suspicion that some of these patients might not even have HCC and received unnecessary LT. However, our analysis did not demonstrate a significant difference in the misdiagnosis rate when comparing this specific category with other subgroups within T2 HCC (Table 3). Surprisingly, neither the largest tumor diameter, number of tumor nodules, nor total tumor diameter correlated with the risk for HCC misdiagnosis.
A significant finding of the present study relates to the very short waiting time (median wait time of 1.7 months) in the study population who did not receive LRT prior to LT. For hepatic nodules with equivocal radiographic characteristics, observing for interval growth over time may help differentiate HCC from benign hepatic nodules. In this regard, short waitlist time may be associated with a greater probability for misdiagnosis. On the other hand, there appeared to be regional and center variations in the rate of misdiagnosis independent of wait time. For example, Region 3 accounted for 40% of all cases of HCC misdiagnosis, whereas Region 10 (similarly short wait time) had significantly fewer patients with misdiagnosis. Additionally, of centers performing at least one LT without LRT, 9% (7/81) had multiple patients with misdiagnosis, whereas nearly 70% of centers did not have a single patient with HCC misdiagnosis. While significant regional variation in wait time exists across the United States, it is possible that HCC misdiagnosis is associated with center-specific practices and not just be a reflection of short wait time. The recent implementation of a mandatory wait of 6 months, intended to reduce the disparities in the LT rate between HCC vs non-HCC patients,19 may also reduce the rate of unnecessary LT by allowing more observation time for ascertaining the diagnosis of HCC. The recommended use of LRT when the waiting time is expected to be at least 6 months20 to bridge HCC patients to LT may also be beneficial from a diagnostic standpoint. Transarterial chemoembolization, for example, may provide angiographic evidence supporting the diagnosis of HCC.
Variables predicting HCC misdiagnosis in this study include younger age at LT listing, AFP <10 ng/mL and non-hepatitis C etiology. Half of patients with age <50 years at LT listing (19/38) who received LT for HCC were found to have no HCC on explant. The profound impact of increasing age on HCC development regardless of etiology of liver disease has been well-established in epidemiologic studies,21,22 and HCC develops rarely before the age of 50. Young patient age <50 years should therefore be taken into account when considering whether or not a patient truly has HCC. It is not surprising to observe a higher prevalence of a normal AFP in patients without HCC on explant. Nevertheless, it is well known that AFP is not a sensitive marker for HCC diagnosis in that normal AFP is seen in up to one-third of patients with HCC, thus limiting the utility of AFP in the screening of HCC.7
One limitation of the present study is the relatively small sample size with T2 HCC who received no LRT before LT, and this hampers both the evaluation of factors associated with HCC misdiagnosis as well as the ability to make clinical predictions based on these risk factors. Our analysis would be biased had we included those with no tumor in the explant as a result of complete response after LRT rather than true misdiagnosis. The strength of this study is the use of a large set of pathologic data on LT patients across all regions. While the study cohort is not necessarily representative of the HCC population as a whole given the lack of LRT and relatively short observed wait time to LT, it is somewhat reassuring that the overall rate of HCC misdiagnosis of 11% in the present study is lower than the previously reported 20–25% misdiagnosis rate before standardized radiographic criteria for HCC were in place.4,23
In summary, among patients undergoing LT with MELD exception for T2 HCC who did not receive LRT, 11% were found to have no HCC on explant. The incidence of misdiagnosis did not differ before and after LI-RADS implementation and was disproportionally high in one UNOS region. More efforts are still needed to eliminate unnecessary LT for patients without HCC.
Acknowledgments
Funding information
This work was supported in part by the Biostatistics Core of the UCSF Liver Center (P30 DK026473).
Footnotes
AUTHORS’ CONTRIBUTIONS
Mehta, Roberts, Hirose, and Yao: Conceive and designed the study; Mehta, Dodge, Roberts, Hirose, and Yao: Acquire, analyzed, or interpret the data; Mehta, Dodge, and Yao: Drafted the manuscript; Mehta, Roberts, Hirose, and Yao: Revised the manuscript; Mehta, Dodge, and Yao: Performed statistical analysis; Hirose, Roberts, and Yao: Supervised.
DISCLOSURES
The authors declare no conflict of interests.
References
- 1.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 2.Halazun KJ, Patzer RE, Rana AA, et al. Standing the test of time: outcomes of a decade of prioritizing patients with hepatocellular carcinoma, results of the UNOS natural geographic experiment. Hepatology. 2014;60:1957–1962. doi: 10.1002/hep.27272. [DOI] [PubMed] [Google Scholar]
- 3.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 4.Freeman RB, Mithoefer A, Ruthazer R, et al. Optimizing staging for hepatocellular carcinoma before liver transplantation: a retrospective analysis of the UNOS/OPTN database. Liver Transpl. 2006;12:1504–1511. doi: 10.1002/lt.20847. [DOI] [PubMed] [Google Scholar]
- 5.Mehta N, Sarkar M, Dodge JL, Fidelman N, Roberts JP, Yao FY. Intention to treat outcome of T1 hepatocellular carcinoma with the “wait and not ablate” approach until meeting T2 criteria for liver transplant listing. Liver Transpl. 2016;22:178–187. doi: 10.1002/lt.24360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao FY, Bass NM, Nikolai B, et al. A follow-up analysis of the pattern and predictors for dropout among patients with hepatocellular carcinoma awaiting liver transplantation: implications for the current organ allocation policy. Liver Transpl. 2003;9:684–692. doi: 10.1053/jlts.2003.50147. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llovet J, Ducreux M, Lencioni R, et al. EASL-EORTC clinical practice guidelines management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell DG, Bruix J, Sherman M, Sirlin CB. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology. 2015;61:1056–1065. doi: 10.1002/hep.27304. [DOI] [PubMed] [Google Scholar]
- 10.Wald C, Russo MW, Heimbach JK, Hussain HK, Pomfret EA, Bruix J. New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology. 2013;266:376–382. doi: 10.1148/radiol.12121698. [DOI] [PubMed] [Google Scholar]
- 11.Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974;9:716–723. [Google Scholar]
- 12.Lindsey JK, Jones B. Choosing among generalized linear models applied to medical data. Stat Med. 1998;17:59–68. doi: 10.1002/(sici)1097-0258(19980115)17:1<59::aid-sim733>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 13.Harper AM, Edwards E, Washburn WK, Heimbach J. An early look at the Organ Procurement and Transplantation Network explant pathology form data. Liver Transpl. 2016;22:757–764. doi: 10.1002/lt.24441. [DOI] [PubMed] [Google Scholar]
- 14.Rahman WT, Hussain HK, Parikh ND, Davenport MS. Reinterpretation of outside hospital MRI abdomen examinations in patients with cirrhosis: is the OPTN mandate necessary? Am J Roentgenol. 2016;207:782–788. doi: 10.2214/AJR.16.16209. [DOI] [PubMed] [Google Scholar]
- 15.Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97–104. doi: 10.1002/hep.21966. [DOI] [PubMed] [Google Scholar]
- 16.Krinsky GA, Lee VS, Theise ND, et al. Transplantation for hepatocellular carcinoma and cirrhosis: sensitivity of magnetic resonance imaging. Liver Transpl. 2002;8:1156–1164. doi: 10.1053/jlts.2002.35670. [DOI] [PubMed] [Google Scholar]
- 17.van den Bos IC, Hussain SM, Dwarkasing RS, et al. MR imaging of hepatocellular carcinoma: relationship between lesion size and imaging findings, including signal intensity and dynamic enhancement patterns. J Magn Reson Imaging. 2007;26:1548–1555. doi: 10.1002/jmri.21046. [DOI] [PubMed] [Google Scholar]
- 18.Samoylova ML, Dodge JL, Mehta N, Yao FY, Roberts JP. Evaluating the validity of model for end-stage liver disease exception points for hepatocellular carcinoma patients with multiple nodules <2 cm. Clin Transplant. 2015;29:52–59. doi: 10.1111/ctr.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heimbach JK, Hirose R, Stock PG, et al. Delayed hepatocellular carcinoma model for end-stage liver disease exception score improves disparity in access to liver transplant in the United States. Hepatology. 2015;61:1643–1650. doi: 10.1002/hep.27704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clavien PA, Lesurtel M, Bossuyt PM, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11–e22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flemming JA, Yang JD, Vittinghoff E, Kim WR, Terrault NA. Risk prediction of hepatocellular carcinoma in patients with cirrhosis: the ADRESS-HCC risk model. Cancer. 2014;120:3485–3493. doi: 10.1002/cncr.28832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guyot E, Sutton A, Rufat P, et al. PNPLA3 rs738409, hepatocellular carcinoma occurrence and risk model prediction in patients with cirrhosis. J Hepatol. 2013;58:312–318. doi: 10.1016/j.jhep.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi PH, Trotter JF, Forman L, et al. Impact of pretransplant diagnosis of hepatocellular carcinoma on cadaveric liver allocation in the era of MELD. Liver Transpl. 2004;10:42–48. doi: 10.1002/lt.20020. [DOI] [PubMed] [Google Scholar]