Highlights
-
•
BCG-primed, Ad5-85A boosted cattle present Ag85A specific mycobacteriocidal T cells.
-
•
Along with mycobacteriocidal activity Ad5-85A boosting induces IL-10 transcription.
-
•
Ad5-85A boosting does not alter the transcription pattern of pro-inflammatory cytokines.
Keywords: Mycobacteria killing, Antigen specific, T-cell lines, Cytokines
Abstract
There is a need to improve the efficacy of the BCG vaccine against human and bovine tuberculosis. Previous data showed that boosting bacilli Calmette-Guerin (BCG)-vaccinated cattle with a recombinant attenuated human type 5 adenovirally vectored subunit vaccine (Ad5-85A) increased BCG protection and was associated with increased frequency of Ag85A-specific CD4+ T cells post-boosting. Here, the capacity of Ag85A-specific CD4+ T cell lines – derived before and after viral boosting – to interact with BCG-infected macrophages was evaluated. No difference before and after boosting was found in the capacity of these Ag85A-specific CD4+ T cell lines to restrict mycobacterial growth, but the secretion of IL-10 in vitro post-boost increased significantly. Furthermore, cell lines derived post-boost had no statistically significant difference in the secretion of pro-inflammatory cytokines (IL-1β, IL-12, IFNγ or TNFα) compared to pre-boost lines. In conclusion, the protection associated with the increased number of Ag85A-specific CD4+ T cells restricting mycobacterial growth may be associated with anti-inflammatory properties to limit immune-pathology.
1. Introduction
Bovine tuberculosis (bTB), caused mainly by Mycobacterium bovis, poses economical, animal welfare and human health problems [1]. Development of vaccines for cattle, to be used in conjunction with current regulations, is part of control and eradication strategies for England and Wales for controlling bTB [2], [3].
Cattle vaccinated with the live attenuated M. bovis bacillus Calmette-Guerin (BCG) and boosted with adenovirus type 5 (Ad5) expressing Mycobacterium tuberculosis Ag85A (Ag85A) (Ad5-Ag85A) showed improved protection against pathology associated with M. bovis [4], [5]. Recently, we adapted the use of the antigen-unbiased T cell expansion method from Geiger et al. [6] to the bovine system [7]. This approach avoids initial in vitro bias caused by expansion of T cell lines by specific repeated cycles of antigen stimulation. We reported that boosting BCG-vaccinated cattle with Ad5-85A increased the frequency of Ag85A-specific CD4+ T cell lines, which correlated with protection, but there was no change in T-cell antigen avidity or epitope-recognition repertoire; the avidity of Ag-85A specific CD4+ T cells was not modulated by viral boosting [7]. Therefore, it was of interest to further characterise the functional properties of these Ag85A-specific CD4+ T cell lines derived from BCG-primed and Ad5-85A-boosted cattle.
In this study, the capacity of these Ag85A-specific CD4+ T cells – generated either before or after Ad-85A boost – to control mycobacteria in vitro and their cytokine profile, after culture for 24 h with BCG-infected macrophages, have been evaluated. Our data suggest that boosting BCG with Ad5-85A enhances protection by increasing the number of Ag85A-specific CD4+ T cells capable of controlling mycobacteria, whilst also potentially developing anti-inflammatory properties to limit immune-pathology.
2. Materials and methods
2.1. Animals
Experiments were carried out according to the UK Animal (Scientific Procedures) Act 1986 under project license PPL70/7737. The study protocol was approved by the APHA Animal Use Ethics Committee (UK Home Office PCD number70/6905) and has been reported previously [5]. Briefly, all animals were vaccinated with 1 × 106 Colony Forming Units (CFU) M. bovis BCG Danish 1331 subcutaneously at week (wk) 0; Ad5-85A boosted cattle were inoculated at wk 8 with 2 × 109 infectious units of Ad5-85A by intradermal injection on the shoulder; all animals were challenged endobronchially with 2 × 103 CFU M. bovis AF2122/97 strain at wk 12 [5]. Peripheral blood mononuclear cells (PBMC) were cryo-preserved pre- (wk 8) and post-boost (wk 11) and used to generate CD4+ T cell lines. The present study utilised Ag85A-specific CD4+ T cell lines, from three BCG-primed Ad5-85A-boosted cattle and one BCG-vaccinated control, acquired in the study described previously [7]. Thirteen pre-boost cell lines were used from two animals (three from one animal and ten from the BCG control) and thirteen post-boost cell lines were used from three animals (five from one animal and four from each of the remaining animals).
2.2. Isolation and selection of pre-/post- boost Ag85A-specific CD4+ T cell lines
Polyclonal CD4+ T cell libraries were generated from pre-boost (wk 8) and post-boost (wk 11) PBMC using a method adapted from Geiger et al. [6], as described previously [7]. Ag85A-specific CD4+ T cells were identified by screening the different polyclonal cell cultures for their capacity to proliferate using 1 × 105 − 2 × 105 CD4+ T cells per culture and 5 µg/ml (initial screening) or 10 µg/ml (subsequent screening) recombinant Ag85A (Lionex GmbH, Germany) and 5 × 103 CD14+ as antigen presenting cells per well of 96-well U-bottom plates. Ag85A-specific CD4+ T cell lines were expanded, after each 11 day Ag85A-selective culture, using 1 µg/ml lectin from Phaseolus vulgaris leucoagglutinin PHA-L (PHA – Sigma-Aldrich) in the presence of 10 U/ml recombinant human interleukin 2 (Gentaur, Belgium) and CD14+ feeder cells for nine days and cryopreserved. All Ag85A-specific CD4+ T cell lines used in these experiments had undergone three sequential rounds of Ag85A-PHA stimulation.
2.3. Bovine monocyte/macrophage cell culture
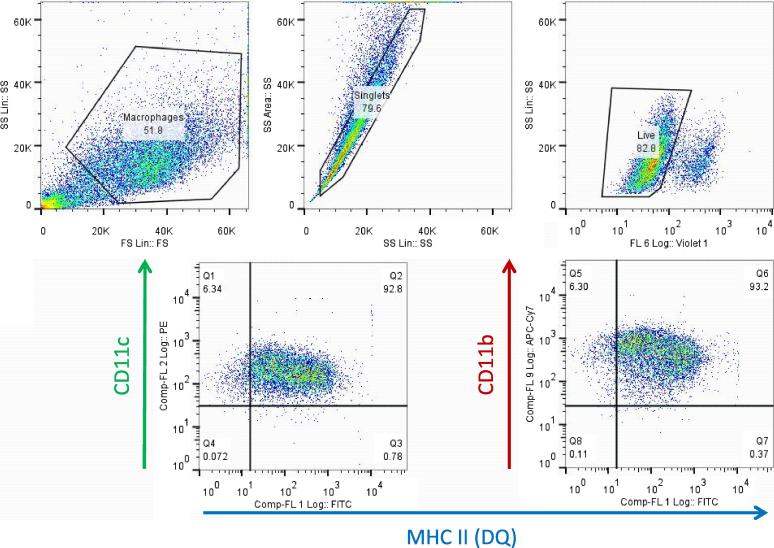
Autologous bovine CD14+ (monocytes) or granulocyte-macrophage colony stimulating factor (GM-CSF)-matured CD14+ cells (macrophages [Mφ]) were cultured at 37 °C (5% CO2) in complete medium consisting of RPMI 1640 containing 2 mM GlutaMax, 25 mM HEPES, 0.1 mM non-essential amino acids, 5 × 10−5 M β-mercaptoethanol, 50 µg/ml Gentamicin (all from Life Technologies, UK), and 10% foetal calf serum (FCS) (Sigma-Aldrich, UK) (complete medium). For Mφ differentiation, monocytes were cultured at a density of 1 × 106/ml in medium containing recombinant bovine GM-CSF diluted 1/100 (Bio-rad, UK) for six days in Corning Ultra-low adhesion flasks (Sigma-Aldrich); cells were fed GM-CSF on day three. After six days, Mφ were harvested by incubating flasks in ice for 20–30 min and washed. Fig. 1 shows a representative immune phenotype profile of GM-CSF matured cells, which defines their surface phenotype as CD11b+CD11c+BoLA-DQ+, which is consistent with GM-CSF matured CD14+/bone-marrow-derived cells [8], [9]). Mφ were labelled with the following murine monoclonal antibodies (mab): mAb CC158 (Bio-rad), against bovine MHC class II (DQ), conjugated to Fluorescein using the Lightning Link kit (Innova Biosciences, UK); IL-A16 (a kind gift from Dr Jan Naessens, ILRI, Nairobi, Kenya) against bovine CD11c, conjugated to R-Phycoerythrin using the Zenon labelling kit (Life Technologies); and mAb CC94, to bovine CD11b, conjugated to Alexa Fluor 700 using the Zenon labelling kit (Life Technologies). Labelled cells were acquired in a CyAn ADP cytometer (Beckman Coulter, UK) using Summit 4.3 (Beckman Coulter) and analysed with FlowJo 7.6.5 (TreeStar, USA). Live cells for analysis were selected based on their FSC/SSC profile and stain pattern with LIVE/DEAD Fixable Violet Dead Cell Stain (Life technologies).
Fig. 1.
Characterisation of CD14+cell-derived mature macrophage phenotype. Representative plots of CD14+ cells matured into macrophages (Mφ) by culturing in medium supplemented with GM-CSF for six days as described in materials and methods. Harvested Mφ were selected based on their large FSC/SSC phenotype and live Mφ were analysed for CD11c, CD11b and MHC class II (DQ) as described. Plots shown were derived from GM-CSF-matured CD14+ obtained from a cow infected naturally with M. bovis that was used to develop the methodology.
2.4. BCG-activation of Ag85A-specific CD4± T cells
BCG Tokyo (a kind gift from Dr M. Behr, McGill University, Canada) was grown as described previously [10]. Prior experiments had indicated that transcription of potentially cytotoxic genes by antigen-specific stimulated CD4+ T-cells occurs at six days after stimulation. Therefore, CD4+ T-cells were stimulated as previously described [13] with small modifications; briefly, monocytes seeded in 24-well plates at a density of 2.5 × 105/ml (1 ml/well), were infected with BCG Tokyo in antibiotic-free medium at a ratio of 4:1 BCG:monocytes. After 16 h, 1 ml of 7.5 × 105 Ag85A-specific CD4+ T cells in complete medium were added to BCG-infected monocytes. Cultures were fed with 10 U/ml IL-2 on day four and used for evaluation of mycobacterial growth inhibition on day six.
2.5. Evaluation of mycobacterial growth inhibition
BCG has been established as an accepted microorganism for the evaluation of mycobacterial growth [11], [12]. Prior to infection with BCG, Mφ were washed in antibiotic-free medium. 2 × 105 Mφ per ml were infected with BCG Tokyo in antibiotic-free medium at a BCG:Mφ ratio of 5:1 in 48-well plates (0.5 ml/well). After 16 h, wells containing BCG-infected Mφ were washed thrice with 0.5 ml HBSS (Gibco) and co-cultured for 24 h with BCG-activated CD4+ T cells, at a density of 8 × 105 cells/ml in a final volume of 0.5 ml of medium containing 50 µg/ml gentamicin; control wells contained infected Mφ but no CD4+ T cells. Supernatants were cryopreserved for analysis and cells were incubated with 0.55 ml/well sterile deionised H2O on ice for 30 mins and disrupted by pipetting; 0.5 ml/well of homogenate was transferred to Mycobacteria Growth Indicator tubes (MGIT) containing 7 ml BBL MGIT broth and 0.8 ml BACTEC MGIT Growth Supplement/BBL MGIT PANTA antibiotic mixture and incubated in a BACTEC MGIT 320 reader (Becton, Dickinson and Company, USA). CFU were calculated using the time to positivity against a BCG Tokyo growth curve. Reduction in BCG CFU was calculated relative to the medium culture control for each animal.
2.6. Analysis of cytokine secretion
Supernatants from the mycobacterial growth inhibition experiments were analysed for IFN-γ secretion using the Bovigam™ assay kit (Prionics AG, Switzerland); IL-1β, IL-10, TNF-α and IL-12 were evaluated using a MULTI-SPOT® 96-Well – 7 Spot plate kit (Meso Scale Discovery (MSD), USA) (IL-10 and IL-12 were a kind gift from Dr J. Hope, then at the Institute for Animal Health, Compton). Concentrations for cytokines were derived from standard curves of recombinant bovine cytokines after subtracting animal-specific background in BCG-infected macrophages.
2.7. Gene transcription assays
RNA was prepared from Ag85A-specific CD4+ T cell lines at days zero and six after BCG-activation, using TRIzol (Ambion, Life Technologies) and RNeasy kits (Qiagen, UK) according to the manufacturers’ instructions. Due to restrictions in the number of cells available for each cell line, it was possible to carry out transcription analysis for six pre-boost cell lines derived from two animals (two from one animal and four from the other) and for four post-boost cell lines derived from three animals (two from one animal and one from each of the remaining animals). cDNA was synthesised from 100 ng total RNA using SuperScript VILO cDNA synthesis kits (Invitrogen) and a GeneAmp PCR System 9700 (Applied Biosystems) at 42 °C for 1 h and 85 °C for 5 min. Bovine perforin, granulysin, granzyme A and GAPDH gene transcription were detected using 2 µl of the cDNA template and 1 µl Taqman Gene expression Assay (20x) primer in a 20 µl Taqman Fast Advanced Mastermix Real-Time PCR reaction (Applied Biosystems, UK) using a ViiA Real-time PCR System (2 min 50 °C hold, 20 sec 95 °C hold, 40 cycles of 1 sec 95 °C denature and 20 sec anneal/extend). Relative BCG-stimulated gene trasncription was determined using the ΔΔCt method with GAPDH as the reference gene. Data shown are for day six relative to day zero. Taqman assay reference codes for bovine perforin, granulysin, granzyme A and GAPDH are provided in Table 1 (Thermo Fisher).
Table 1.
ThermoFisher RT-PCR Taqman assay kit reference codes.
| Perforin | Bt03816262_m1 |
| Granulysin | Bt03287460_g1 |
| Granzyme | Bt03271024_m1 |
| GAPDH | Bt03210914_g1 |
2.8. Statistics
Data analysis was performed using GraphPad Prism version 7 (GraphPad Software, La Jolla, CA, USA). Comparisons were made using the unpaired two-tailed t-test with significance level set at 5%.
3. Results
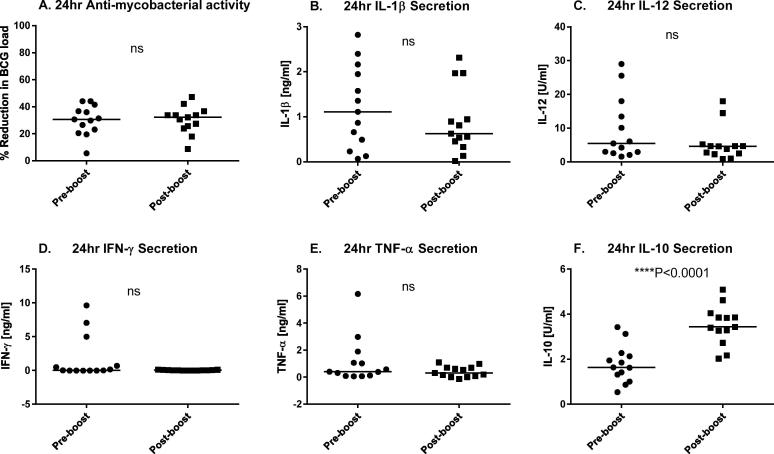
Ag85A-specific CD4+ T cell lines derived from BCG-vaccinated cattle, before and after boosting with Ad5-85A (generated and cyro-preserved in a previous study [7]), were evaluated for recognition of BCG-infected autologous Mφ in a 24 h co-culture system adapted from Endsley et al. [13]. Mycobacterial growth inhibition was assessed by determining the reduction of BCG load after co-culture (Fig. 2A). No difference in the ability to inhibit mycobacterial growth was found between Ag85A-specific CD4+ T cells lines obtained prior to and after boost, suggesting that boosting BCG-vaccinated cattle with Ad5-85A does not increase the anti-mycobacterial capacity of Ag85A-specific CD4+ T cells. Similarly, no statistically significant differences were found in the secretion of the pro-inflammatory cytokines IL-1β, IL-12, IFN-γ and TNF-α (Fig. 2B–E) between cultures containing pre-boost and post-boost Ag85A-specific CD4+ T cells. Nevertheless, there were common trends towards lower amounts of pro-inflammatory cytokines in post-boost cultures. In contrast, we observed a statistically significant increase in IL-10 secretion in the cultures containing Ag85A-specific CD4+ T cells obtained post-boost (Fig. 2F) compared to the IL-10 levels induced in Ag85A-specific CD4+ T cells obtained pre-boost.
Fig. 2.
Comparison of the anti-mycobacterial capacity and cytokine response of activated Ag85A-specific CD4+ T cell lines derived from BCG-primed Ad5-85A-boosted cattle. Ag85A-specific CD4+ T cell lines derived from BCG-vaccinated cattle, both pre- and post- boosting with Ad5-85A, were stimulated with BCG-infected monocytes for six days and co-cultured for 24 h with BCG-infected Mφ as described. The percentage reduction in BCG load (A) was analysed as described in materials and methods, together with cytokine secretion: IL-1β (B), IL-12 (C), IFN-γ (D), TNF-α (E) and IL-10 (F). The thirteen pre-boost cell lines were derived from two animals (three from one animal and ten from a BCG control) and the thirteen post-boost cell lines were derived from three animals (four from one animal and five from each of the remaining animals). Statistical comparisons were made using the unpaired t-test.
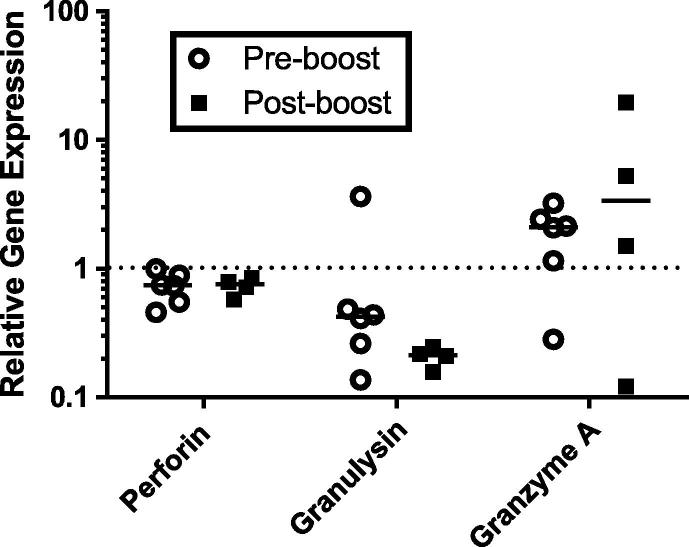
The observed lack of difference in anti-mycobacterial capacity of Ag85A-specific CD4+ T cell lines ex vivo is consistent with comparable pre- and post-boost transcription of effector molecules thought to be involved in the killing of mycobacteria, (perforin, granulysin and granzyme A, [Fig. 3]).
Fig. 3.
Evaluation of transcription of cytotoxic genes in BCG-activated Ag85A-specific CD4+ T cell lines derived from BCG-primed Ad5-85A boosted cattle. Ag85A-specific CD4+ T cell lines derived from BCG-vaccinated cattle, both pre- and post- boosting with Ad5-85A, were activated with BCG-infected CD14+ cells for six days as described in materials and methods. RNA was extracted from Ag85A-specific CD4+ T cells at day zero and day six after BCG-stimulation and analysed for bovine perforin, granulysin and granzyme A gene expression. Due to restrictions in the number of cells available for each cell line, it was possible to carry out transcription analysis for six pre-boost cell lines derived from two animals (two from one animal and four from the other) and for four post-boost cell lines derived from three animals (two from one animal and one from each of the remaining animals). Data shown are for transcription observed at day six relative to transcription observed at day zero.
4. Discussion
In this study, boosting BCG vaccinated cattle with Ad5-85A did not induce significant differences in Ag85A-specific CD4+ T cell lines obtained post-boost compared to cell lines obtained prior to boosting in terms of secretion of pro-inflammatory cytokines, transcription of molecules associated with cytotoxicity, or control of mycobacteria. However, Ag85A-specific CD4+ T cell lines obtained post-boost secreted more of the immune regulatory cytokine IL-10 than CD4+ T cell lines obtained prior to viral boosting.
It has been proposed that the induction of robust Th1 responses is a strategy employed by mycobacteria to facilitate its multiplication and dissemination [14], [15], [16], [17], [18]. More recently, Moguche et al. [19] showed in mice that continuous expression of ESAT-6 drives the expansion and maintenance of ESAT-6 specific T-cells. This continuous expansion resulted in large numbers of terminally differentiated ESAT-6 specific T-cells with limited protective capacity. In contrast, expression of Ag85 is limited to the early stages of infection, resulting in the priming of Ag85A/Ag85B specific memory T-cells which, although functional, are reduced in numbers due to the limited amount of Ag85A present during infection in later stages of infection.
It is likely that the level of protection observed in BCG vaccinated and Ad5-85A boosted cattle was in part due to the expansion of the pool of cells specific for Ag85A [7]. This increase in the number of functionally active Ag85A-specific T-cells prior to infection enabled Ag85A to be detected early during infection, when Ag85A is more likely to be expressed by mycobacteria. Thus, we speculate that it is the availability of a greater number of Ag85A-specific CD4+T-cells, induced by boosting (as previously shown [7]), which led to a greater control of mycobacteria (rather than any enhancement in anti-mycrobacterial mechanisms which we did not detect). However, the control of mycobacteria by Ag85A-specific CD4+ T-cells appears to be accompanied by the production of IL-10; this is somewhat surprising as IL-10 has been associated with a decrease in protection against mycobacteria [20], [21]. On the other hand IL-10 is an immune-regulatory/anti-inflammatory cytokine that contributes to the control of IFNγ responses which, whilst necessary for protection against mycobacteria, have been positively associated with pathology induced by M. bovis [22]. In this work the selection of CD4+ cells was based on their proliferative response, first in an unbiased manner by stimulation with PHA and then in an antigen specific manner by stimulation with Ag85A. It has been shown that cells detected in proliferative assays, such as the one used in this work, do not necessarily correlate with other types of assays such as those detecting production of IFNγ (Steinbach, S. et al unpublished observation). Thus it is possible that protection induced by Ad5-Ag85A may be the result of the induction of two functionally different Ag85A-specific CD4+ T-cell populations; one population producing inflammatory cytokines, which were able to control, directly or indirectly, mycobacteria; and one population producing IL-10, with anti-inflammatory properties capable of controlling pathology. Successful vaccination against mycobacteria requires the control of the pathogen and of the detrimental immune response induced by the pathogen. The data obtained from these experiments contribute to our understanding of the nature of the immune response required for protection against tuberculosis in cattle. This information is required for the formulation of more rationally designed vaccines capable of inducing protective immune responses whilst avoiding the induction of pathogenic immune responses.
Funding
This work was funded by a BBSRC grant (BB/K010018/1); the animal experiment from which material was derived was funded by Defra project SE3224. HMV is a Jenner Investigator. The funding body did not have a role on the study design, collection, analysis or interpretation of the data or the writing of the manuscript or in the decision to publish the manuscript.
Conflict of interests
None. Hannah J. Metcalfe adapted the CD4+ T cell methods to the experiments, performed the experiments and wrote the paper; Lucia Biffar contributed to the design and implementation of the MGIT analysis; Sabine Steinbach optimised the bovine CD4+ T cell library/avidity methods and reviewed/edited the paper; Efraín Guzmán provided advice in the generation of monocyte derived macrophages and macrophage staining; Tim Connelley was involved in design of the project and contributed to experimental planning; W. Ivan Morrison was involved in design of the project, contributed to experimental planning and reviewed/edited the paper; Bernardo Villarreal-Ramos and Martin Vordermeier were both involved in design of the project, contributed to experimental planning, provided advice in the setting up of the system and reviewed/edited the paper. All authors approved the final version of the submitted manuscript.
References
- 1.Krebs JR, Anderson R, Clutton-Brock T, Morrison I, Young D, Donnelly C. Bovine tuberculosis in cattle and badgers – report to the Rt Hon Dr Jack Cunningham MP. The Ministry of Agriculture FaF; 1997.
- 2.Defra. The strategy for achieving officially bovine tuberculosis free status for England. In: Defra, editor. England, UK: Defra; 2014.
- 3.Welsh-Government. Bovine TB eradication programme. Wales, UK: Welsh Government; 2017.
- 4.Vordermeier H.M., Villarreal-Ramos B., Cockle P.J., McAulay M., Rhodes S.G., Thacker T. Viral booster vaccines improve Mycobacterium bovis BCG-induced protection against bovine tuberculosis. Infect Immun. 2009;77(8):3364–3373. doi: 10.1128/IAI.00287-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dean G., Whelan A., Clifford D., Salguero F.J., Xing Z., Gilbert S. Comparison of the immunogenicity and protection against bovine tuberculosis following immunization by BCG-priming and boosting with adenovirus or protein based vaccines. Vaccine. 2014;32(11):1304–1310. doi: 10.1016/j.vaccine.2013.11.045. [DOI] [PubMed] [Google Scholar]
- 6.Geiger R., Duhen T., Lanzavecchia A., Sallusto F. Human naive and memory CD4+ T cell repertoires specific for naturally processed antigens analyzed using libraries of amplified T cells. J Exp Med. 2009;206(7):1525–1534. doi: 10.1084/jem.20090504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metcalfe H.J., Steinbach S., Jones G.J., Connelley T., Morrison W.I., Vordermeier M. Protection associated with a TB vaccine is linked to increased frequency of Ag85A-specific CD4(+) T cells but no increase in avidity for Ag85A. Vaccine. 2016;34(38):4520–4525. doi: 10.1016/j.vaccine.2016.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norimatsu M., Harris J., Chance V., Dougan G., Howard C.J., Villarreal-Ramos B. Differential response of bovine monocyte-derived macrophages and dendritic cells to infection with Salmonella typhimurium in a low-dose model in vitro. Immunology. 2003;108(1):55–61. doi: 10.1046/j.1365-2567.2003.01557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helft J., Bottcher J., Chakravarty P., Zelenay S., Huotari J., Schraml B.U. GM-CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c(+)MHCII(+) macrophages and dendritic cells. Immunity. 2015;42(6):1197–1211. doi: 10.1016/j.immuni.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Villarreal-Ramos B., Berg S., Chamberlain L., McShane H., Hewinson R.G., Clifford D. Development of a BCG challenge model for the testing of vaccine candidates against tuberculosis in cattle. Vaccine. 2014;32(43):5645–5649. doi: 10.1016/j.vaccine.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheon S.H., Kampmann B., Hise A.G., Phillips M., Song H.Y., Landen K. Bactericidal activity in whole blood as a potential surrogate marker of immunity after vaccination against tuberculosis. Clin Diagn Lab Immunol. 2002;9(4):901–907. doi: 10.1128/CDLI.9.4.901-907.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fletcher H.A., Tanner R., Wallis R.S., Meyer J., Manjaly Z.R., Harris S. Inhibition of mycobacterial growth in vitro following primary but not secondary vaccination with Mycobacterium bovis BCG. Clin Vaccine Immunol. 2013;20(11):1683–1689. doi: 10.1128/CVI.00427-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endsley J.J., Hogg A., Shell L.J., McAulay M., Coffey T., Howard C. Mycobacterium bovis BCG vaccination induces memory CD4+ T cells characterized by effector biomarker expression and anti-mycobacterial activity. Vaccine. 2007;25(50):8384–8394. doi: 10.1016/j.vaccine.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Coscolla M., Copin R., Sutherland J., Gehre F., de Jong B., Owolabi O. M. tuberculosis T cell epitope analysis reveals paucity of antigenic variation and identifies rare variable TB antigens. Cell Host Microbe. 2015 doi: 10.1016/j.chom.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodworth J.S., Andersen P. Reprogramming the T cell response to tuberculosis. Trends Immunol. 2016;37(2):81–83. doi: 10.1016/j.it.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orme I.M., Robinson R.T., Cooper A.M. The balance between protective and pathogenic immune responses in the TB-infected lung. Nat Immunol. 2015;16(1):57–63. doi: 10.1038/ni.3048. [DOI] [PubMed] [Google Scholar]
- 17.Woodworth J.S., Aagaard C.S., Hansen P.R., Cassidy J.P., Agger E.M., Andersen P. Protective CD4 T cells targeting cryptic epitopes of Mycobacterium tuberculosis resist infection-driven terminal differentiation. J Immunol. 2014;192(7):3247–3258. doi: 10.4049/jimmunol.1300283. [DOI] [PubMed] [Google Scholar]
- 18.Andersen P., Woodworth J.S. Tuberculosis vaccines–rethinking the current paradigm. Trends Immunol. 2014;35(8):387–395. doi: 10.1016/j.it.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Moguche A.O., Musvosvi M., Penn-Nicholson A., Plumlee C.R., Mearns H., Geldenhuys H. Antigen availability shapes T cell differentiation and function during tuberculosis. Cell Host Microbe. 2017;21(6) doi: 10.1016/j.chom.2017.05.012. 695–706 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreira-Teixeira L., Redford P.S., Stavropoulos E., Ghilardi N., Maynard C.L., Weaver C.T. T cell-derived IL-10 impairs host resistance to mycobacterium tuberculosis infection. J Immunol. 2017;199(2):613–623. doi: 10.4049/jimmunol.1601340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redford P.S., Boonstra A., Read S., Pitt J., Graham C., Stavropoulos E. Enhanced protection to Mycobacterium tuberculosis infection in IL-10-deficient mice is accompanied by early and enhanced Th1 responses in the lung. Eur J Immunol. 2010;40(8):2200–2210. doi: 10.1002/eji.201040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vordermeier H.M., Chambers M.A., Cockle P.J., Whelan A.O., Simmons J., Hewinson R.G. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect Immun. 2002;70(6):3026–3032. doi: 10.1128/IAI.70.6.3026-3032.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]