Abstract
Parkinson disease (PD) is a common neurodegenerative disorder, which involves degeneration of dopaminergic neurons that are present in the substantia nigra pars compacta (SNpc) region. Many factors have been identified that could lead to Parkinson disease, however almost all of them are directly or indirectly dependent on Ca2+ signaling. Importantly, though disturbances in Ca2+ homeostasis have been implicated in Parkinson’s disease and other neuronal diseases, the identity of the calcium channel remains elusive. Members of the transient receptor potential canonical (TRPC) channel family have been identified as a new class of calcium channels and it could be anticipated that these channels could play a major role in neurodegenerative diseases, especially in PD. Thus, in this chapter we have entirely focused our attention on TRPC channels and elucidating its role in PD.
Introduction
Calcium (Ca2+) is an important element that functions as a prominent regulator for processes such as transcription and gene regulation, neuronal cell growth and differentiation, motility and axonal development and even neuronal cell death (Berridge et al., 2003; Bollimuntha et al., 2011; Sun et al., 2015). Thus, it is not surprising that disruption of Ca2+ homeostasis in neuronal cells results in decreased neuronal functions leading to neurodegenerative diseases such as Parkinson’s, Huntington’s and Alzheimer’s (Albers and Beal, 2000; Bollimuntha et al., 2005; Cali et al., 2011; O’Bryant et al., 2009; Racette et al., 2006; Reboreda et al., 2011; Ruat and Traiffort, 2013; Zuccato and Cattaneo, 2007, 2009). Due to these outcomes, Ca2+ homeostasis is strictly maintained in neuronal cells. Cells have evolved a multitude of mechanisms to regulate cellular Ca2+ levels and Ca2+ channels and pumps play a key role in this. Cumulative studies suggest that both excessive elevation and attenuation of intracellular Ca2+ will lead to neuronal degeneration albeit through different mechanisms as suggested in this chapter (Figure 1). Increased intracellular calcium [Ca2+]i concentrations mainly via the AMPA or NMDA channels lead to enhanced activation of Ca2+ dependent processes that are normally inert or functional at low Ca2+ levels, thereby causing metabolic imbalances which result in neuronal death (Arundine and Tymianski, 2003; Choi, 1988; Herman et al., 1990). In contrast decreased [Ca2+]i, upon store depletion and the focus of this chapter, could induce ER stress or inhibit activation of proteins that are essential for cell survival (Paschen, 2000). Thus, different actions of Ca2+ in neuronal cells could be dependent not only on its cellular concentration but also on the ion channels that modulate Ca2+ entry (Naziroglu, 2011; Paschen, 2000; Yuruker et al., 2015). Ca2+ channels, mainly the transient receptor potential canonical (TRPC) channels, have recently emerged as a key regulator of Ca2+ homeostasis in neuronal cell function (Bollimuntha et al., 2011; Selvaraj et al., 2010). Further, TRPC channel function and expression are altered in various neuronal diseases such as Parkinson’s disease (PD) (Badger et al., 2014; Selvaraj et al., 2009; Selvaraj et al., 2010). PD is a neurodegenerative disorder, which includes progressive degeneration of dopaminergic neurons and effects the aging population. Thus, in this chapter we have focused on the functional implication of TRPC channels in neuronal cell function and their role in PD.
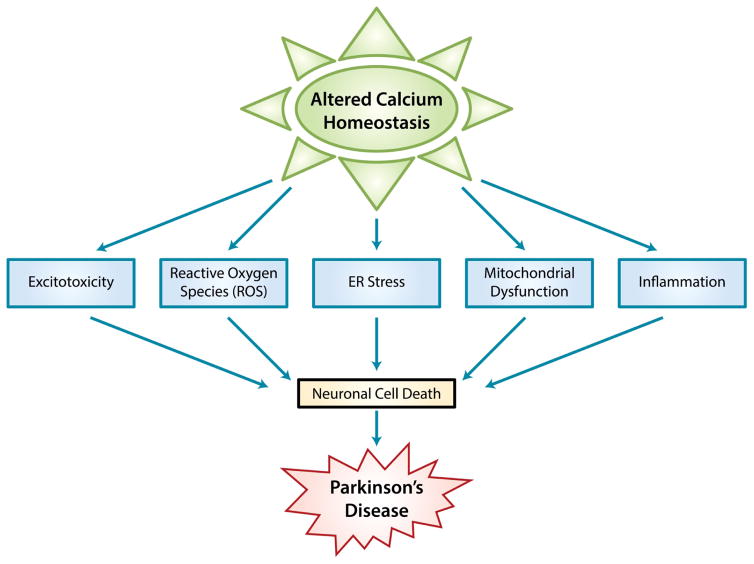
Figure 1. Mechanisms that leads to PD.
Schematic model showing altered Ca2+ homeostasis in the neuronal cells which causes Parkinson’s disease via various mechanism(s) such as ER stress, ROS, mitochondrial dysfunction, excitotoxicity, and inflammation as shown in the model..
TRPC channels and Ca2+ signaling
The transient receptor potential (TRP) channel superfamily is one of the largest families of non-selective cation channels (Nilius and Owsianik, 2011) that are activated through diverse mechanism(s). The TRP superfamily is divided into various subfamilies which are TRPC (canonical), TRPM (melastatin), TRPV (vanilloid), TRPP (polycystin), TRPML (mucolipin), TRPA (ankyrin) and TRPN (NOMPC-like, which is found only in invertebrates) (Nilius, 2007)(Abramowitz and Birnbaumer, 2009; Pan et al., 2011; Sukumaran et al., 2012). TRP channels have been identified as candidates involved in regulating cellular functions ranging from pure sensory function to molecular regulation and survival, suggesting that these channels could serve as gatekeepers for transcellular transport of monovalent and divalent cations such as Na+ and Ca2+ ions (Flockerzi, 2007; Löf et al., 2011; Nilius and Owsianik, 2011). In addition, most of the TRPs, especially TRPCs, have been shown to multimerize to form either homo- or heteromeric functional channels that have unique channel properties (Nilius and Owsianik, 2011).
TRPCs are Ca2+-permeable nonselective cation channels in the subfamily of TRP channels. In addition, TRPCs have the highest degree of homology to the first discovered Drosophila photoreceptors’ TRP channels (Kiselyov et al., 2010) and have been suggested as candidates for the store-operated Ca2+ entry channels. Seven members of the TRPC subfamily (TRPC1-7) have been identified and all of them are highly expressed in neuronal cells, with some of the TRPC channels expressing predominantly in neuronal cells (Bollimuntha et al., 2011; Sun et al., 2014). All TRPCs (with the exception of TRPC2 which is a pseudogene in humans) are functional and play an important role in modulating calcium homeostasis. Based on sequence analysis (protein homology) TRPC channels could be again sub divided into four sub groups as they share similar protein sequences: TRPC1, TRPC2, TRPC3/6/7, and TRPC4/5 (Venkatachalam and Montell, 2007). Although the crystal structure of any of the TRPC proteins have yet to be determined, domain predictions suggest that these channels have six transmembrane domains with both N- and C-termini facing intracellularly (Flockerzi, 2007; Ong et al., 2014; Venkatachalam and Montell, 2007). Importantly, both the N and C-terminus have also been shown to interact with many signaling proteins that could contribute to their regulation as well as could modulate neuronal function (Gaudet, 2008).
Functions attributed to TRPC in Neurons
Neuronal cells are heavily dependent on Ca2+ signaling for their function and for their survival (Komuro and Kumada, 2005). Initiation of the action potential activates the voltage gated calcium channels that modulate neurosecretion. In addition, channels such as NMDA, AMPA and others are also present on the post-synaptic membranes that induce Ca2+ entry to modulate neuronal functions. Finally, another mechanism that could have a role in neuronal function has recently been identified to initiate Ca2+ entry, but the mechanism involves the depletion of internal ER. Growing evidence suggest that this mode of Ca2+ entry is also essential in maintaining the cytosolic, ER and mitochondrial Ca2+ levels. Importantly, as TRPC proteins have been identified as putative calcium channels that are activated by second messenger-mediated store depletion, they could play a critical role in neuronal survival, proliferation, and differentiation. This is also evident as TRPCs are highly expressed in all regions of the central nervous system (CNS) (Huang et al., 2011), but in some cases the expression of individual TRPC channels is altered during development, especially as observed with TRPC1 and TRPC3, which are more expressed in embryonic CNS than in adult neurons (Strübing et al., 2003). Furthermore, neuronal growth in the presence of growth factors, such as basic fibroblast growth factor (bFGF), was dependent on Ca2+ entry through TRPC channels, especially TRPC1, 4, but not TRPC5, suggesting their important role in neuronal growth and survival (Fiorio Pla et al., 2005). Importantly, hindering the function of TRPC channels or silencing of TRPC1 alone decreased bFGF-induced intracellular Ca2+ increase and proliferation of neuronal stem cells (Li et al., 1999; Montecinos-Oliva et al., 2014; Su et al., 2014). TRPC3 has also been shown to be associated with BDNF receptors regulating the stimulation of neuronal growth (Hirschler-Laszkiewicz et al., 2009; Tong et al., 2008) and Epo-persuade cell differentiation and proliferation (Mattson et al., 1989). Consistent with these results, expression of TRPC1, TRPC2, and TRPC4 was observed to be higher, whereas expression of TRPC6 was decreased in neuronal stem cells. This differential expression of various TRPC channels could suggest that different TRPC channels could play contrasting role during neuronal cell proliferation and differentiation (Su et al., 2014). In addition, using hippocampal neuronal cells (H19-7), Wu and their colleagues showed that Ca2+ influx through TRPC1 and TRPC3 were essential in regulating the shift between proliferation and differentiation (Wu et al., 2004b).
Importantly, Ca2+ entry is not always beneficial. Activation by glutamate massively increases [Ca2+]i mainly through TRPC1 channels that leads to cell death as observed in hippocampal organotypic slice cultures. TRPC1 expression was enhanced after glutamate treatment and both inhibition of TRPC channel by 2APB or knockdown of TRPC1 significantly reduced cell death, indicating that TRPC1 could activate glutamate-induced cell death in the hippocampus (REF). In contrast, studies have also shown that physiological activation of TRPC1 through other G-protein receptors could protect the neurons from several extracellular stimuli (Dhar et al., 2014; Li et al., 2012; Wu et al., 2004). Similarly, loss of Ca2+ entry has also shown to decrease endoplasmic reticulum (ER) Ca2+ levels inducing ER stress and promoting cell death (Selvaraj et al., 2012). Together these studies suggest that TRPC channels could have a dual role where normal activation of TRPC channels regulates neuronal development and excessive Ca2+ influx, as observed upon glutamate treatment, can induce neuronal degeneration (Figure 2). In addition, there appears to be a set-point for Ca2+ entry, where physiologic concentration of calcium is beneficial and both excessive and decreased calcium entry is harmful. Consistent with this notion, it has been shown that equally high Ca2+ loads are toxic when entering through the NMDA channels, but not when entering through the voltage-dependent Ca2+ channels (Ovey and Naziroglu, 2015; Strübing et al., 2001) suggesting that the Ca2+ channel(s) are critical in deciding the fate of the neuron. The other possibility could be that as TRPC forms multimers where different subunits of known TRPC channels could associate and could have a completely different role. Consistent with this notion, TRPC1, TRPC4 and TRPC5 are observed to be highly expressed in the pyramidal cell layer of the hippocampus, frontal cortex and dentate gyrus, while TRPC6 is dispersedly stained only at the molecular layer of the dentate gyrus (Chung et al., 2006; Strübing et al., 2001). Furthermore, both TRPC1 and TRPC3 were observed to protect hippocampal cell lines and silencing of either of these channels inhibited cell development and proliferation (Wu et al., 2004). In contrast, both TRPC1 and TRPC6 are found in the substantia nigra region where it co-localizes with tyrosine hydroxylase (TH)-positive neurons, as well as with mGluR1, suggesting their role in modulating dopaminergic neurons (Bollimuntha et al., 2011; Fendt et al., 2008). Selective degeneration of hippocampal and other neurons is a common feature in several neurodegenerative conditions including Alzheimer’s disease, epilepsy, Huntington, stroke and Parkinson. Accumulating evidence suggests that glutamate, an excitatory neurotransmitter is intimately involved in neurodegeneration (Phelan et al., 2013); however the identification of a particular TRPC channels is not yet established. In addition, TRPC channels could also function as a scaffold protein where it could regulate other proteins. This function would be independent of their ability to regulate Ca2+ influx, since most TRPC have been shown to form large protein-protein complexes. Thus, more research is needed to fully establish the role of TRPC channels in neuronal function and identify if alterations in their expression vs their ability to bring calcium entry modulates neurodegeneration.
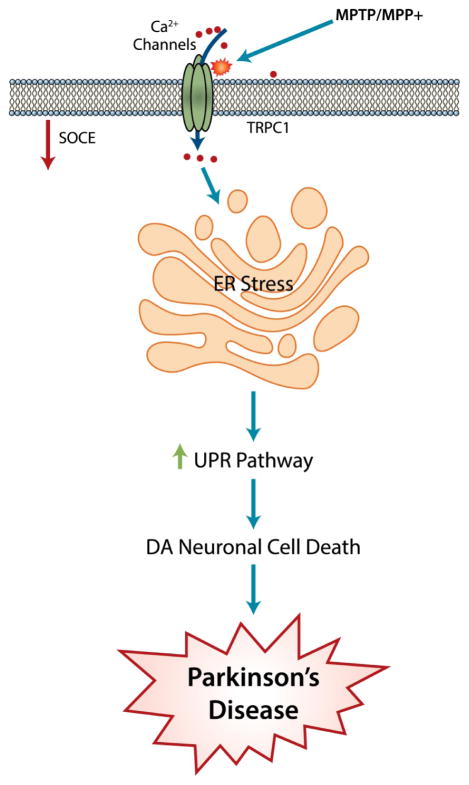
Figure 2. Role of TRPC proteins in PD.
proposed model for MPP+/MPTP-induced DA loss which could lead to the onset/progression of PD. MPP+/MPTP attenuates the expression of TRPC1 and SOC-mediated Ca2+ influx that leads to prolonged ER Ca2+ depletion and activation of the UPR pathways and subsequent ER stress–mediated neurodegeneration which leads to many neuronal disease like PD.
Role of TRPC channels in Parkinson’s disease
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, which is caused by the progressive loss of dopaminergic (DA) neurons present in the substantia nigra pars compacta (SNpc) region. Degeneration of DA neurons in the SNpc region is the reason for the observed loss of motor symptoms with this disease (Javitch et al., 1985). Although the mechanism as to why these neurons are specifically degenerated is not well known, a host of pathogenic factors have been suggested to be responsible for the degeneration of dopaminergic neurons in the SNpc. These factors include: mitochondrial dysfunction, ER and oxidative stress, protein aggregation, excitotoxicity, and inflammation where each factors could individually or collectively play a role in the pathogenesis of PD (Figure 1). In addition, lysosomal-mediated degradation has recently been suggested to inhibit the protein degradation pathway that could eventually lead to protein aggregation as observed in PD. The role of calcium regulation in PD has been of interest as it overlaps with several of the established pathways that lead to neurodegeneration. Importantly, as discussed above, changes in [Ca2+]i could either stimulate intracellular events that could trigger or inhibit cell death process (Berridge et al., 2000; Putney, 2003). Increases in [Ca2+]i via the Cav1.3 channels has been shown to be necessary to stimulate the release of dopamine (DA) from dopaminergic neurons of the SNpc (Chen and Rice, 2001; Patel et al., 2009). Interestingly, Cav1.3 channels were highly expressed in samples obtained from PD patients, indicating the importance of Ca2+ in PD. Furthermore, disturbances in Ca2+ homeostasis have been implicated in Parkinson’s disease (Albers and Beal, 2000; Bollimuntha et al., 2005; Cali et al., 2011; O’Bryant et al., 2009; Zuccato and Cattaneo, 2007; Zuccato and Cattaneo, 2009). As many factors involved in neuronal function are dependent on Ca2+ signaling, it could be anticipated that loss of these critical functions could contribute to PD (Berridge et al., 2000; Putney, 2003). In addition, [Ca2+]i is maintained by the removal of Ca2+ through the activity of plasma membrane Ca2+-ATPase pump and the Na+/Ca2+ exchanger or sequestration into intracellular organelle stores by the sarco-endoplasmic reticulum ATPase pump. However, most of these require ATP for functionality and as ATP levels are decreased in PD, it is possible that activity of these proteins could also lead to PD, though more research is needed to fully establish the role of calcium signaling machinery in PD. In addition, decreases in ATP levels could decrease ER calcium levels that could stimulate store-operated Ca2+ entry which is dependent on TRPC and Orai channels. Thus, it is critical to establish there physiological function as discussed in this chapter.
TRPC1
TRPC1, the founding member of canonical TRPCs, is ubiquitously expressed including high expression in all neuronal tissues (Ambudkar et al., 2006; Strübing et al., 2003; Tozzi et al., 2003). Alterations in intracellular Ca2+, especially in the storage organelles such as the ER and mitochondria have also been shown to affect neuronal survival and are closely linked with PD (Kazuno et al., 2006). Although several Ca2+ channels have been identified that bring Ca2+ inside the cells, Ca2+ entry through the store-operated channels (SOC) could be the most important. Moreover, as Ca2+ entry through SOC channels has been shown to be essential for maintaining intracellular ER Ca2+ stores, along with regulating many cellular functions, they could play an important role in neuronal survival (Bollimuntha et al., 2006; Selvaraj et al., 2012; Sun et al., 2014) TRPC1 has been shown to be essential for the formation of the functional SOC channel as it is activated by store depletion per se in DA neurons (Bollimuntha et al., 2006; Selvaraj et al., 2012; Selvaraj et al., 2009; Sun et al., 2014; Wang and Poo, 2005).
One of the possible mechanisms that could lead to neurodegeneration in PD could be due to the initiation of ER stress or the unfolded protein response (UPR), but the mechanism is not well known (Hoozemans et al., 2007; Hoozemans et al., 2012). Moreover, abnormal protein aggregation has been identified as one of the causes of PD, but the mechanism is not well defined. Ca2+ in the ER is known to be important for protein synthesis and folding, thus loss of this vital function could induce abnormal protein aggregation as well as ER stress that could activate cell death cascades (Putney, 2005; Yoshida et al., 2006). Ca2+ influx through the SOCE mechanism is essential for the refilling of the ER Ca2+ stores, which could prevent abnormal protein aggregation and ER stress. Recently published data (Bollimuntha et al., 2006; Selvaraj et al., 2012; Selvaraj et al., 2009; Arshad et al., 2014) suggests that in dopaminergic neurons TRPC1 functions as the endogenous plasma membrane SOC Ca2+ channel. Importantly, the expression of TRPC1, but not that of other TRPCs as well as ORAI1 or SOCE modulator stromal interaction molecule 1 (STIM1), was decreased in cell and animal models as well in PD patients. Interestingly, Ca2+ entry into dopaminergic cells was also inhibited upon neurotoxin treatment that induced PD-like symptoms and ER Ca2+ levels were also decreased. Consistent with these results, overexpression of TRPC1 reduced neurotoxicity induced by MPP+ or salsolinol (Bollimuntha et al., 2006; Selvaraj et al., 2012). In contrast, knockdown of TRPC1 or by the addition of TRPC channel blockers, inhibited dopaminergic cell survival, indicating that some of the protection exhibited by TRPC1 might be contributed by its Ca2+ influx ability. Similar results were also found in vivo model and in tissue samples from human patients. Loss of TRPC1 also showed a decrease in ER Ca2+ levels and initiated the unfolded protein response. Moreover, overexpression of functional TRPC1 protected against neurotoxin-induced loss of SOCE and the resultant ER stress response. In contrast, silencing of TRPC1 or its modulator STIM1 increased the UPR, which revealed that ER stress is activated in PD and TRPC1 could decreased ER stress (Selvaraj et al., 2012) (Figure 2). Furthermore, Ca2+ entry via TRPC1 also activated AKT/mTOR signaling and contributes to neuronal survival (Selvaraj et al., 2012).
Mitochondrial dysfunction is another mechanism that has been demonstrated to have a role in the PD pathogenesis (Moon and Paek, 2015). Importantly, Calcium entry via SOC channels have been shown to modulate mitochondrial Ca2+ levels and alterations in these Ca2+ levels could also lead to neurodegeneration. In addition, a strong correlation between SOCE and apoptosis has also been suggested, indicating that lack of Ca2+ entry initiates apoptosis (Jayadev et al., 1999). Neurotoxins induce neuronal loss by decreasing TRPC1 levels, which could decrease mitochondrial Ca2+ levels necessary for ATP synthesis followed by disrupting mitochondrial membrane potential and initiation of apoptosis. Consistent with these results, activation of TRPC1 restored mitochondrial membrane potential and inhibited Bax translocation to the mitochondria thus preventing cytochrome c release and mitochondrial-mediated apoptosis. These results suggest that TRPC1 could prevent neurotoxin induced cellular death by preserving the loss of mitochondrial membrane potential, which in turn inhibits degenerative apoptotic signaling to provide neuroprotection (Bollimuntha et al., 2006; Bollimuntha et al., 2005; Selvaraj et al., 2009). In contrast, another study showed that downregulation of STIM1 expression, which is known to activate TRPC channels, inhibited apoptotic cell death and reduced intracellular ROS production in PC12 cells that were treated with 6-hydroxydopamine (Li et al., 2014). Although TRPC channels were not evaluated in this study, mitochondrial dysfunction, loss of mitochondrial membrane potential and the decrease of ATP generation was observed in STIM1 knockdown cells that were treated with 6-hydroxydopamine (Li et al., 2014). One possibility could be that PC12 cells are very different than dopaminergic cells and STIM1 has been shown to modulate other calcium channels that could account for these discrepancies.
TRPC2
Among the seven different members of the TRPC, the TRPC2 channel, being a pseudogene in human is the least investigated member of the TRPC channels (Löf et al., 2011; Tornquist et al., 2014). Therefore, relatively little is known in regard to its physiological significance. In rodents, TRPC2 is highly localized to the dendritic tip of the vomeronasal sensory neurons (Kiselyov et al., 2010; Yildirim and Birnbaumer, 2007) and hence plays a significant role in pheromone sensing in rodents (Flanagan et al., 2011; Kimchi et al., 2007); however its role in PD is not yet identified.
TRPC3
TRPC3 is highly expressed in brain and with regard to PD, increased Ca2+ homeostasis has been linked to altered oxidative stress levels that could lead to neuronal loss. Oxidative stress has also been shown to regulate TRPC3 channels, where TRPC3 channels are directly activated in response to oxidative stress. The oxidant tertiary butyl hydroperoxide completely depolarized endothelial cells by the activation TRPC3 (Selvaraj et al., 2010), suggesting that TRPC3 determines endothelial redox sensitivity. In addition, over expression of TRPC3 in HEK293T cells showed an increase in basal membrane conductance upon tertiary butyl hydroperoxide treatment, which was mainly due to the influx of Na+ (Poteser et al., 2006). In another study in primary rat cortical neurons and astrocytes, TRPC3 levels and TRPC3-mediated Ca2+ fluxes was dose-dependently decreased upon treatment with oxidative stressors (Roedding et al., 2013). In addition, in murine striatal astrocytes addition of neurotoxins, that mimic PD, decreased ATP as well as OAG-induced Ca2+ transients and wave formations, suggesting that TRPC3 could be essential for these functions (Streifel et al., 2014). Importantly, a slight increase in TRPC3 expression was observed in PD condition as well as neurotoxin models of PD (Selveraj et al., 2012). Disruption of Ca2+ signaling especially in astrocyte could significantly impact neuronal function and survival especially during neurological injury and in disease conditions such as PD. Together these studies indeed suggest that TRPC3 dysfunction is involved in Ca2+ and oxidative stress signaling observed in PD.
Parkinsonian movement disorders are also associated with abnormalities in SN pars reticulata (SNr) (Lee et al., 2007; Nevet et al., 2004; Rivlin-Etzion et al., 2006; Wichmann and Soares, 2006). TRPC3 channels are expressed in SNr GABA projection neurons, where TRPC3 are tonically active and mediate a voltage-independent inward current, leading to a substantial depolarization in these neurons (Zhou et al., 2008). Inhibition of TRPC3 channels induce hyperpolarization, decrease firing frequency, and increase firing irregularity demonstrating that TRPC3 channels play critical roles in maintaining the depolarized membrane potential, high firing frequency, and firing regularity in these basal ganglia output neurons crucial to Parkinsonian movement disorders (Zhou et al., 2008). In addition, dopamine release from the dendrites excited SNr GABA neurons via the dopamine receptors that could activate TRPC3 channels, to mediate an inward, Na+-dependent current, leading to a substantial depolarization and ensuring appropriate firing intensity and pattern in SNr GABA projection neurons (Zhou et al., 2010). TRPC3 channels have also been shown to modulate motor coordination (Becker et al., 2009; Hartmann et al., 2008). In an ataxic mouse mutant (moonwalker, Mwk mice) that displays motor and coordination defects, a gain-of-function mutation (T635A) in TRPC3 channels was observed. As a result sustained activation of TRPC3 channels was observed that diminished dendritic arborization and lead to the progressive loss of Purkinje neurons. Similarly, another study also showed the loss of TRPC3 exhibits atrophy and progressive paralysis suggesting an essential role of TRPC3 channels in neuronal signaling, differentiation and development (Rodriguez-Santiago et al., 2007; Hartmann et al., 2008). Although none of these studies looked at dopaminergic neurons, it could be still suggestive that abnormal TRPC3 function may also contribute to Parkinson’s disease which is classified as loss of motor function.
TRPC4
TRPC4α/β isoforms are the most abundantly expressed and functionally characterized in brain. TRPC4 and TRPC5 are the major TRPC subtypes in the adult rat brain because both are expressed highly in the pyramidal cell layer of the hippocampus, frontal cortex and dentate gyrus (Chung et al., 2006; Strübing et al., 2001). TRPC4 is specifically detected throughout the layers (2–6) of the prefrontal cortex or the motor cortex (Huang et al., 2007; Morelli et al., 2013). Although the role of TRPC4 in PD is not yet defined, its role in axonal regeneration in adult rat dorsal root ganglia (DRG) has been reported (Wu et al., 2008). The expression of TRPC4 was enhanced whereas TRPC1, TRPC3, TRPC6 and TRPC7 expression remained unchanged after nerve injury persuaded by either sciatic nerve transection or intra-ganglionic microinjection of dibutyryl cAMP (Selvaraj et al., 2010; Wu et al., 2008). In addition, TRPC4 expression in various neuronal cells has been shown to be increased upon addition of NGF and dibutyryl cAMP that induced differentiation (Selvaraj et al., 2010; Wu et al., 2008). Suppression of TRPC4 by specific small interfering RNA significantly reduced the length of neuritis in cultured DRG neurons (Wu et al., 2008). Taken together, these results suggest that TRPC4 contributes to axonal regeneration especially after nerve injury. If these findings have generality, TRPC4 could be an important molecular target for potential regeneration therapies in patients suffering from neuronal injury. In contrast, by using whole-genome sequencing a recent report showed that gain-of-function mutations in TRPC4 gene induces cell death in dopamine neurons through a defined, calcium-related downstream pathway (Nagarajan, et al., 2014). High expression of TRPC4 is also found in the PM of soma and proximal dendrites of lateral septal neurons, colocalizing with mGlu receptors, which could also contribute to cell death. Studies also shows that TRPC4 is expressed in cells from the ventral tegmental area, a region with extensive inputs from dopamine neurons which are important in regulating the animal behavior (Huang et al., 2011). Importantly, a recent report has shown that self-administration of cocaine was significantly less in the TRPC4 KO group than WT controls (Klipec et al., 2016). Also, spontaneous dopamine neuronal activity in the ventral tegmental area revealed fewer cells with high-frequency firing rate in rats that lack TRPC4. Together these studies show the significance of TRPC4 channels in various functions of the CNS making them an important molecular target, especially for neurological diseases associated with excitotoxicity like PD or drug addiction that are also dependent on dopaminergic neurons.
TRPC5
Studies by March et al. showed that TRPC5 along with TRPC1 is highly expressed in substantia nigra and TRPC5 is localized in the neuronal cells, thereby suggesting a significant role of TRPC5 channels in neurons (De March et al., 2006). In neurite outgrowth, TRPC5 showed a vital role since the dominant negative pore mutant of TRPC5 inhibits and overexpression of TRPC5 enhances the neurite outgrowth of the transfected neurons (Kumar et al., 2012). Studies showed that a balance of TRPC5, TRPC1 and TRPC6 expression regulates the neurite extension rate in neuronal cells (Kumar et al., 2012). In another study, they showed that calpain, a calcium activated protease, cleaves and activates TRPC5 channels to participate in semaphoring 3A-induced neuronal growth cone collapse (Kaczmarek et al., 2012). Recent studies by Jemal et al. 2014 detailed how increased expression of TRPC5 at the PM enhances the proportion of neuronal cells that respond to hyposomotic stimulation. They suggested that hypoosmotic cell-swelling activates Gq-coupled receptors, which in turn stimulates the activation of TRPC5 (Jemal et al., 2014). TRPC5 also play a vital role as a switch between proliferation and neuronal differentiation in neural progenitor cells (NPCs) (Shin et al., 2010). TRPC5 channels have been shown to have an inhibitory role on neurite extension (Bezzerides et al., 2004; Tian et al., 2010). Puram et al showed that TRPC5 couples Ca2+ signaling to an ubiquitin ligase pathway at the centrosome and thereby orchestrates dendrite patterning and connectivity in the brain (Puram et al., 2011) Thereby, TRPC5 could play a vital role in normal motor coordination and gait in mice. TRPC5 has been found to form a specific complex with CaMKIIβ, but not CaMKIIα. This complex then initiates activation of CaMKIIβ which leads to the phosphorylation and inhibition of the ubiquitin ligase Cdc20-APC at the centrosome (Puram et al., 2011). Recent studies by Hong et al showed that TRPC5 S-glutathionylation by oxidative stress and attenuated TRPC1 expression contributed to neuronal damage in the striatum. Loss of striatum is very common feature in many neurodegenerative diseases, like Huntington’s disease (Hong et al., 2015). To support their finding they also showed that a TRPC5 blocker enriched rearing behavior in Huntington’s disease transgenic mice. These finding suggest that TRPC5 channels may have important developmental functions in addition to their sensory receptive roles in mature neurons and could play a significant role in many neuronal disease including PD.
TRPC6
TRPC6 is shown to play a vital role in ischemic brain damage. Studies by Du et al showed that in rats TRPC6 inhibition suppressed ischemic brain damage. The expression of TRPC6 protein in ischemia was specifically downregulated by the NMDA receptor–dependent calpain proteolysis which blocked its degradation and protected the neurons and brains against cerebral ischemia (Du et al., 2010). A following study by the same group showed that TRPC6 attenuates the NMDA receptor and activities and protects the neuronal cells from ischemic excitotoxicity. They also showed that the infarct volume in TRPC6 transgenic (Tg) mice was reduced as compared to wild-type (WT) littermates (Li et al., 2012a). An interesting connection between TRPC6 and depression has been suggested since TRPC6 is activated by hyperforin, an antidepressant extract (Tu et al., 2010). TRPC6 has also been suggested to have a significant role in AD. Transient expression of presenilin (PS) mutant, a gene linked to early-onset of Alzheimer’s disease, with TRPC6 in HEK-239 cells resulted in a strong inhibition of agonist induced Ca2+ entry (Lessard et al., 2005). Recent studies by Kim and Kang showed a significant role of TRPC6 in seizure exposure and seizure-related neuronal damage in the rat denate gyrus. In their study, they showed that TRPC6 expression is attenuated in chronic epileptic rats and a TRPC6 knockdown increased the seizure susceptibility in dentate gyrus of wildtype rats (Kim and Kang, 2015). Another recent study by Chauvet et al, showed that the Na+/K+-ATPase and the amyloid-beta peptide aβ1-40 regulates the activity of TRPC6 channels (Chauvet et al., 2015). The study suggested that the abundance, distribution and activity of TRPC6 can be regulated by amyloid peptide, which is known to increase in PD patients, hence this study clearly indicate a significant role of TRPC6 channels in PD.
TRPC7
Recently studies showed a critical role of TRPC7 in initiation of seizures induced by pilocarpine both in vitro and in vivo (Phelan et al., 2014) owing to the importance of TRPC7 in neuronal diseases like PD, but broad understanding of the role TRPC7 in neuronal physiology remains elusive. In the proliferating rat H19-7 hippocampal neurons, the expression of TRPC7 was enhanced and following differentiation attenuated the expression (Goel et al., 2002). Significant studies have been done on heteromeric dimers of TRPC7 with TRPC3 in neuronal cells. Studies show that TRPC3/C7 are molecular targets for metabotropic glutamate receptor signaling in rat striatal cholinergic interneurons (Berg et al., 2007)
Conclusion
Significant research in the past decades has explored the role of TRPC channels with regard to neuronal survival, differentiation, and neurodegeneration as observed in Parkinson disease. Although several questions still remain unanswered, it could be suggested that Ca2+ influx via the TRPC channels have a significant role in the onset/progression/suppression of neurodegenerative diseases such as Parkinson’s. Thus, targeting Ca2+ entry (both inhibition as well as activation) through these TRPC channels could be critical for maintaining normal physiological function in dopaminergic neurons. Recent findings have also implicated STIM1 as a regulator for SOCE making research in STIM1 function/expression in neuronal cells as an interesting topic as they may be involved in neurological diseases such as Parkinson’s. Although the current data with regard to the function of TRPC in neuronal cells is overwhelming, significant information including that from human and animal tissues is still missing.
Acknowledgments
We thank the grant support from the NIH (DE017102, DE024300-01A1) awarded to B.B.S, and the assistance of John Swift, School of Medicine and Health Sciences in making the figures.
References
- Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009;23:297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers DS, Beal MF. Mitochondrial dysfunction and oxidative stress in aging and neurodegenerative disease. J Neural Transm Suppl. 2000;59:133–154. doi: 10.1007/978-3-7091-6781-6_16. [DOI] [PubMed] [Google Scholar]
- Ambudkar IS, Bandyopadhyay BC, Liu X, Lockwich TP, Paria B, Ong HL. Functional organization of TRPC-Ca2+ channels and regulation of calcium microdomains. Cell calcium. 2006;40:495–504. doi: 10.1016/j.ceca.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–337. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- Badger JL, Cordero-Llana O, Hartfield EM, Wade-Martins R. Parkinson’s disease in a dish - Using stem cells as a molecular tool. Neuropharmacology. 2014;76(Pt A):88–96. doi: 10.1016/j.neuropharm.2013.08.035. [DOI] [PubMed] [Google Scholar]
- Becker EB, Oliver PL, Glitsch MD, Banks GT, Achilli F, Hardy A, Nolan PM, Fisher EM, Davies KE. A point mutation in TRPC3 causes abnormal Purkinje cell development and cerebellar ataxia in moonwalker mice. Proc Natl Acad Sci U S A. 2009;106:6706–6711. doi: 10.1073/pnas.0810599106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AP, Sen N, Bayliss DA. TrpC3/C7 and Slo2.1 are molecular targets for metabotropic glutamate receptor signaling in rat striatal cholinergic interneurons. J Neurosci. 2007;27:8845–8856. doi: 10.1523/JNEUROSCI.0551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Bezzerides VJ, I, Ramsey S, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- Bollimuntha S, Ebadi M, Singh BB. TRPC1 protects human SH-SY5Y cells against salsolinol-induced cytotoxicity by inhibiting apoptosis. Brain Res. 2006;1099:141–149. doi: 10.1016/j.brainres.2006.04.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollimuntha S, Selvaraj S, Singh BB. Emerging roles of canonical TRP channels in neuronal function. Adv Exp Med Biol. 2011;704:573–593. doi: 10.1007/978-94-007-0265-3_31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollimuntha S, Singh BB, Shavali S, Sharma SK, Ebadi M. TRPC1-mediated inhibition of 1-methyl-4-phenylpyridinium ion neurotoxicity in human SH-SY5Y neuroblastoma cells. J Biol Chem. 2005;280:2132–2140. doi: 10.1074/jbc.M407384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cali T, Ottolini D, Brini M. Mitochondria, calcium, and endoplasmic reticulum stress in Parkinson’s disease. BioFactors. 2011;37:228–240. doi: 10.1002/biof.159. [DOI] [PubMed] [Google Scholar]
- Chauvet S, Boonen M, Chevallet M, Jarvis L, Abebe A, Benharouga M, Faller P, Jadot M, Bouron A. The Na+/K+-ATPase and the amyloid-beta peptide abeta1-40 control the cellular distribution, abundance and activity of TRPC6 channels. Biochim Biophys Acta. 2015;1853:2957–2965. doi: 10.1016/j.bbamcr.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Chen BT, Rice ME. Novel Ca2+ dependence and time course of somatodendritic dopamine release: substantia nigra versus striatum. J Neurosci. 2001;21:7841–7847. doi: 10.1523/JNEUROSCI.21-19-07841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988;11:465–469. doi: 10.1016/0166-2236(88)90200-7. [DOI] [PubMed] [Google Scholar]
- Chung YH, Sun Ahn H, Kim D, Hoon Shin D, Su Kim S, Yong Kim K, Bok Lee W, Ik Cha C. Immunohistochemical study on the distribution of TRPC channels in the rat hippocampus. Brain Res. 2006;1085:132–137. doi: 10.1016/j.brainres.2006.02.087. [DOI] [PubMed] [Google Scholar]
- De March Z, Giampa C, Patassini S, Bernardi G, Fusco FR. Cellular localization of TRPC5 in the substantia nigra of rat. Neurosci Lett. 2006;402:35–39. doi: 10.1016/j.neulet.2006.03.061. [DOI] [PubMed] [Google Scholar]
- Dhar M, Wayman GA, Zhu M, Lambert TJ, Davare MA, Appleyard SM. Leptin-induced spine formation requires TrpC channels and the CaM kinase cascade in the hippocampus. J Neurosci. 2014;34:10022–10033. doi: 10.1523/JNEUROSCI.2868-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Huang J, Yao H, Zhou K, Duan B, Wang Y. Inhibition of TRPC6 degradation suppresses ischemic brain damage in rats. The Journal of clinical investigation. 2010;120:3480–3492. doi: 10.1172/JCI43165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Schmid S, Thakker DR, Jacobson LH, Yamamoto R, Mitsukawa K, Maier R, Natt F, Husken D, Kelly PH, McAllister KH, Hoyer D, van der Putten H, Cryan JF, Flor PJ. mGluR7 facilitates extinction of aversive memories and controls amygdala plasticity. Molecular psychiatry. 2008;13:970–979. doi: 10.1038/sj.mp.4002073. [DOI] [PubMed] [Google Scholar]
- Fiorio Pla A, Maric D, Brazer S-C, Giacobini P, Liu X, Chang YH, Ambudkar IS, Barker JL. Canonical transient receptor potential 1 plays a role in basic fibroblast growth factor (bFGF)/FGF receptor-1-induced Ca2+ entry and embryonic rat neural stem cell proliferation. J Neurosci. 2005;25:2687–2701. doi: 10.1523/JNEUROSCI.0951-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan KA, Webb W, Stowers L. Analysis of male pheromones that accelerate female reproductive organ development. PLoS ONE. 2011:6. doi: 10.1371/journal.pone.0016660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flockerzi V. An introduction on TRP channels. Handb Exp Pharmacol. 2007:1–19. doi: 10.1007/978-3-540-34891-7_1. [DOI] [PubMed] [Google Scholar]
- Gaudet R. TRP channels entering the structural era. J Physiol. 2008;586:3565–3575. doi: 10.1113/jphysiol.2008.155812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel M, Sinkins WG, Schilling WP. Selective association of TRPC channel subunits in rat brain synaptosomes. J Biol Chem. 2002;277:48303–48310. doi: 10.1074/jbc.M207882200. [DOI] [PubMed] [Google Scholar]
- Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, Blum R, Dietrich A, Freichel M, Flockerzi V, Birnbaumer L, Konnerth A. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron. 2008;59:392–398. doi: 10.1016/j.neuron.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman B, Gores GJ, Nieminen AL, Kawanishi T, Harman A, Lemasters JJ. Calcium and pH in anoxic and toxic injury. Crit Rev Toxicol. 1990;21:127–148. doi: 10.3109/10408449009089876. [DOI] [PubMed] [Google Scholar]
- Hirschler-Laszkiewicz I, Tong Q, Conrad K, Zhang W, Flint WW, Barber AJ, Barber DL, Cheung JY, Miller BA. TRPC3 activation by erythropoietin is modulated by TRPC6. J Biol Chem. 2009;284:4567–4581. doi: 10.1074/jbc.M804734200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C, Seo H, Kwak M, Jeon J, Jang J, Jeong EM, Myeong J, Hwang YJ, Ha K, Kang MJ, Lee KP, Yi EC, Kim IG, Jeon JH, Ryu H, So I. Increased TRPC5 glutathionylation contributes to striatal neuron loss in Huntington’s disease. Brain: a journal of neurology. 2015;138:3030–3047. doi: 10.1093/brain/awv188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoozemans JJ, van Haastert ES, Eikelenboom P, de Vos RA, Rozemuller JM, Scheper W. Activation of the unfolded protein response in Parkinson’s disease. Biochemical and biophysical research communications. 2007;354:707–711. doi: 10.1016/j.bbrc.2007.01.043. [DOI] [PubMed] [Google Scholar]
- Hoozemans JJ, van Haastert ES, Nijholt DA, Rozemuller AJ, Scheper W. Activation of the unfolded protein response is an early event in Alzheimer’s and Parkinson’s disease. Neuro-degenerative diseases. 2012;10:212–215. doi: 10.1159/000334536. [DOI] [PubMed] [Google Scholar]
- Huang J, Du W, Yao H, Wang Y. TRPC Channels in Neuronal Survival. In: Zhu MX, editor. TRP Channels. Boca Raton (FL): 2011. [PubMed] [Google Scholar]
- Huang WC, Young JS, Glitsch MD. Changes in TRPC channel expression during postnatal development of cerebellar neurons. Cell Calcium. 2007;42:1–10. doi: 10.1016/j.ceca.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Javitch JA, D’Amato RJ, Strittmatter SM, Snyder SH. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci U S A. 1985;82:2173–2177. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayadev S, Petranka JG, Cheran SK, Biermann JA, Barrett JC, Murphy E. Reduced capacitative calcium entry correlates with vesicle accumulation and apoptosis. J Biol Chem. 1999;274:8261–8268. doi: 10.1074/jbc.274.12.8261. [DOI] [PubMed] [Google Scholar]
- Jemal I, Soriano S, Conte AL, Morenilla C, Gomis A. G protein-coupled receptor signalling potentiates the osmo-mechanical activation of TRPC5 channels. Pflugers Archiv: European journal of physiology. 2014;466:1635–1646. doi: 10.1007/s00424-013-1392-z. [DOI] [PubMed] [Google Scholar]
- Kaczmarek JS, Riccio A, Clapham DE. Calpain cleaves and activates the TRPC5 channel to participate in semaphorin 3A-induced neuronal growth cone collapse. Proc Natl Acad Sci U S A. 2012;109:7888–7892. doi: 10.1073/pnas.1205869109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazuno AA, Munakata K, Nagai T, Shimozono S, Tanaka M, Yoneda M, Kato N, Miyawaki A, Kato T. Identification of mitochondrial DNA polymorphisms that alter mitochondrial matrix pH and intracellular calcium dynamics. PLoS genetics. 2006;2:e128. doi: 10.1371/journal.pgen.0020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Kang TC. The role of TRPC6 in seizure susceptibility and seizure-related neuronal damage in the rat dentate gyrus. Neuroscience. 2015;307:215–230. doi: 10.1016/j.neuroscience.2015.08.054. [DOI] [PubMed] [Google Scholar]
- Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–1014. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- Kiselyov K, van Rossum DB, Patterson RL. TRPC channels in pheromone sensing. Vitam Horm. 2010;83:197–213. doi: 10.1016/S0083-6729(10)83008-0. [DOI] [PubMed] [Google Scholar]
- Komuro H, Kumada T. Ca2+ transients control CNS neuronal migration. Cell Calcium. 2005;37:387–393. doi: 10.1016/j.ceca.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Kumar S, Chakraborty S, Barbosa C, Brustovetsky T, Brustovetsky N, Obukhov AG. Mechanisms controlling neurite outgrowth in a pheochromocytoma cell line: the role of TRPC channels. J Cell Physiol. 2012;227:1408–1419. doi: 10.1002/jcp.22855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JI, Verhagen Metman L, Ohara S, Dougherty PM, Kim JH, Lenz FA. Internal pallidal neuronal activity during mild drug-related dyskinesias in Parkinson’s disease: decreased firing rates and altered firing patterns. J Neurophysiol. 2007;97:2627–2641. doi: 10.1152/jn.00443.2006. [DOI] [PubMed] [Google Scholar]
- Lessard CB, Lussier MP, Cayouette S, Bourque G, Boulay G. The overexpression of presenilin2 and Alzheimer’s-disease-linked presenilin2 variants influences TRPC6-enhanced Ca2+ entry into HEK293 cells. Cell Signal. 2005;17:437–445. doi: 10.1016/j.cellsig.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Li H, Huang J, Du W, Jia C, Yao H, Wang Y. TRPC6 inhibited NMDA receptor activities and protected neurons from ischemic excitotoxicity. J Neurochem. 2012a;123:1010–1018. doi: 10.1111/jnc.12045. [DOI] [PubMed] [Google Scholar]
- Li HS, Xu XZ, Montell C. Activation of a TRPC3-dependent cation current through the neurotrophin BDNF. Neuron. 1999;24:261–273. doi: 10.1016/s0896-6273(00)80838-7. [DOI] [PubMed] [Google Scholar]
- Li M, Chen C, Zhou Z, Xu S, Yu Z. A TRPC1-mediated increase in store-operated Ca2+ entry is required for the proliferation of adult hippocampal neural progenitor cells. Cell Calcium. 2012b;51:486–496. doi: 10.1016/j.ceca.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Löf C, Viitanen T, Sukumaran P, Törnquist K. TRPC2: of mice but not men. Adv Exp Med Biol. 2011;704:125–134. doi: 10.1007/978-94-007-0265-3_6. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Guthrie PB, Kater SB. Intrinsic factors in the selective vulnerability of hippocampal pyramidal neurons. Prog Clin Biol Res. 1989;317:333–351. [PubMed] [Google Scholar]
- Montecinos-Oliva C, Schuller A, Parodi J, Melo F, Inestrosa NC. Effects of tetrahydrohyperforin in mouse hippocampal slices: neuroprotection, long-term potentiation and TRPC channels. Current medicinal chemistry. 2014;21:3494–3506. doi: 10.2174/0929867321666140716091229. [DOI] [PubMed] [Google Scholar]
- Moon HE, Paek SH. Mitochondrial Dysfunction in Parkinson’s Disease. Experimental neurobiology. 2015;24:103–116. doi: 10.5607/en.2015.24.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli MB, Amantini C, Liberati S, Santoni M, Nabissi M. TRP channels: new potential therapeutic approaches in CNS neuropathies. CNS Neurol Disord Drug Targets. 2013;12:274–293. doi: 10.2174/18715273113129990056. [DOI] [PubMed] [Google Scholar]
- Naziroglu M. TRPM2 cation channels, oxidative stress and neurological diseases: where are we now? Neurochem Res. 2011;36:355–366. doi: 10.1007/s11064-010-0347-4. [DOI] [PubMed] [Google Scholar]
- Nevet A, Morris G, Saban G, Fainstein N, Bergman H. Discharge rate of substantia nigra pars reticulata neurons is reduced in non-parkinsonian monkeys with apomorphine-induced orofacial dyskinesia. J Neurophysiol. 2004;92:1973–1981. doi: 10.1152/jn.01036.2003. [DOI] [PubMed] [Google Scholar]
- Nilius B. TRP channels in disease. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2007;1772:805–812. doi: 10.1016/j.bbadis.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011:12. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryant SE, Hobson V, Hall JR, Waring SC, Chan W, Massman P, Lacritz L, Cullum CM, Diaz-Arrastia R. Brain-derived neurotrophic factor levels in Alzheimer’s disease. J Alzheimers Dis. 2009;17:337–341. doi: 10.3233/JAD-2009-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong HL, de Souza LB, Cheng KT, Ambudkar IS. Physiological functions and regulation of TRPC channels. Handb Exp Pharmacol. 2014;223:1005–1034. doi: 10.1007/978-3-319-05161-1_12. [DOI] [PubMed] [Google Scholar]
- Ovey IS, Naziroglu M. Homocysteine and cytosolic GSH depletion induce apoptosis and oxidative toxicity through cytosolic calcium overload in the hippocampus of aged mice: involvement of TRPM2 and TRPV1 channels. Neuroscience. 2015;284:225–233. doi: 10.1016/j.neuroscience.2014.09.078. [DOI] [PubMed] [Google Scholar]
- Pan Z, Yang H, Reinach PS. Transient receptor potential (TRP) gene superfamily encoding cation channels. Human genomics. 2011;5:108–116. doi: 10.1186/1479-7364-5-2-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen W. Role of calcium in neuronal cell injury: which subcellular compartment is involved? Brain research bulletin. 2000;53:409–413. doi: 10.1016/s0361-9230(00)00369-5. [DOI] [PubMed] [Google Scholar]
- Patel JC, Witkovsky P, Avshalumov MV, Rice ME. Mobilization of calcium from intracellular stores facilitates somatodendritic dopamine release. J Neurosci. 2009;29:6568–6579. doi: 10.1523/JNEUROSCI.0181-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan KD, Shwe UT, Abramowitz J, Birnbaumer L, Zheng F. Critical role of canonical transient receptor potential channel 7 in initiation of seizures. Proc Natl Acad Sci U S A. 2014;111:11533–11538. doi: 10.1073/pnas.1411442111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan KD, Shwe UT, Abramowitz J, Wu H, Rhee SW, Howell MD, Gottschall PE, Freichel M, Flockerzi V, Birnbaumer L, Zheng F. Canonical transient receptor channel 5 (TRPC5) and TRPC1/4 contribute to seizure and excitotoxicity by distinct cellular mechanisms. Mol Pharmacol. 2013;83:429–438. doi: 10.1124/mol.112.082271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poteser M, Graziani A, Rosker C, Eder P, Derler I, Kahr H, Zhu MX, Romanin C, Groschner K. TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. J Biol Chem. 2006;281:13588–13595. doi: 10.1074/jbc.M512205200. [DOI] [PubMed] [Google Scholar]
- Puram SV, Riccio A, Koirala S, Ikeuchi Y, Kim AH, Corfas G, Bonni A. A TRPC5-regulated calcium signaling pathway controls dendrite patterning in the mammalian brain. Genes & development. 2011;25:2659–2673. doi: 10.1101/gad.174060.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW. Physiological mechanisms of TRPC activation. Pflugers Archiv: European journal of physiology. 2005;451:29–34. doi: 10.1007/s00424-005-1416-4. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr Capacitative calcium entry in the nervous system. Cell Calcium. 2003;34:339–344. doi: 10.1016/s0143-4160(03)00143-x. [DOI] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Marmor O, Heimer G, Raz A, Nini A, Bergman H. Basal ganglia oscillations and pathophysiology of movement disorders. Current opinion in neurobiology. 2006;16:629–637. doi: 10.1016/j.conb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Santiago M, Mendoza-Torres M, Jimenez-Bremont JF, Lopez-Revilla R. Knockout of the trcp3 gene causes a recessive neuromotor disease in mice. Biochemical and biophysical research communications. 2007;360:874–879. doi: 10.1016/j.bbrc.2007.06.150. [DOI] [PubMed] [Google Scholar]
- Roedding AS, Tong SY, Au-Yeung W, Li PP, Warsh JJ. Chronic oxidative stress modulates TRPC3 and TRPM2 channel expression and function in rat primary cortical neurons: relevance to the pathophysiology of bipolar disorder. Brain Res. 2013;1517:16–27. doi: 10.1016/j.brainres.2013.04.025. [DOI] [PubMed] [Google Scholar]
- Selvaraj S, Sun Y, Singh BB. TRPC channels and their implication in neurological diseases. CNS Neurol Disord Drug Targets. 2010;9:94–104. doi: 10.2174/187152710790966650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S, Sun Y, Watt JA, Wang S, Lei S, Birnbaumer L, Singh BB. Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling. The Journal of clinical investigation. 2012;122:1354–1367. doi: 10.1172/JCI61332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S, Watt JA, Singh BB. TRPC1 inhibits apoptotic cell degeneration induced by dopaminergic neurotoxin MPTP/MPP(+) Cell Calcium. 2009;46:209–218. doi: 10.1016/j.ceca.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HY, Hong YH, Jang SS, Chae HG, Paek SL, Moon HE, Kim DG, Kim J, Paek SH, Kim SJ. A role of canonical transient receptor potential 5 channel in neuronal differentiation from A2B5 neural progenitor cells. PLoS ONE. 2010;5:e10359. doi: 10.1371/journal.pone.0010359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem. 2003;278:39014–39019. doi: 10.1074/jbc.M306705200. [DOI] [PubMed] [Google Scholar]
- Su B, Ji Y-S, Sun X-l, Liu X-H, Chen Z-Y. Brain-derived neurotrophic factor (BDNF)-induced mitochondrial motility arrest and presynaptic docking contribute to BDNF-enhanced synaptic transmission. J Biol Chem. 2014;289:1213–1226. doi: 10.1074/jbc.M113.526129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran P, Lof C, Kemppainen K, Kankaanpaa P, Pulli I, Nasman J, Viitanen T, Tornquist K. Canonical transient receptor potential channel 2 (TRPC2) as a major regulator of calcium homeostasis in rat thyroid FRTL-5 cells: importance of protein kinase C delta (PKCdelta) and stromal interaction molecule 2 (STIM2) J Biol Chem. 2012;287:44345–44360. doi: 10.1074/jbc.M112.374348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Sukumaran P, Bandyopadhyay BC, Singh BB. Physiological Function and Characterization of TRPCs in Neurons. Cells. 2014;3:455–475. doi: 10.3390/cells3020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Sukumaran P, Schaar A, Singh BB. TRPM7 and its role in neurodegenerative diseases. Channels (Austin) 2015;9:253–261. doi: 10.1080/19336950.2015.1075675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian D, Jacobo SMP, Billing D, Rozkalne A, Gage SD, Anagnostou T, Pavenstädt H, Pavenstaedt H, Hsu H-H, Schlondorff J, Ramos A, Greka A. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci Signal. 2010:3. doi: 10.1126/scisignal.2001200. [DOI] [PMC free article] [PubMed]
- Tong Q, Hirschler-Laszkiewicz I, Zhang W, Conrad K, Neagley DW, Barber DL, Cheung JY, Miller BA. TRPC3 is the erythropoietin-regulated calcium channel in human erythroid cells. J Biol Chem. 2008;283:10385–10395. doi: 10.1074/jbc.M710231200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornquist K, Sukumaran P, Kemppainen K, Lof C, Viitanen T. Canonical transient receptor potential channel 2 (TRPC2): old name-new games. Importance in regulating of rat thyroid cell physiology. Pflugers Archiv: European journal of physiology. 2014;466:2025–2034. doi: 10.1007/s00424-014-1509-z. [DOI] [PubMed] [Google Scholar]
- Tu P, Gibon J, Bouron A. The TRPC6 channel activator hyperforin induces the release of zinc and calcium from mitochondria. J Neurochem. 2010;112:204–213. doi: 10.1111/j.1471-4159.2009.06446.x. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GX, Poo MM. Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature. 2005;434:898–904. doi: 10.1038/nature03478. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Soares J. Neuronal firing before and after burst discharges in the monkey basal ganglia is predictably patterned in the normal state and altered in parkinsonism. J Neurophysiol. 2006;95:2120–2133. doi: 10.1152/jn.01013.2005. [DOI] [PubMed] [Google Scholar]
- Wu D, Huang W, Richardson PM, Priestley JV, Liu M. TRPC4 in rat dorsal root ganglion neurons is increased after nerve injury and is necessary for neurite outgrowth. J Biol Chem. 2008;283:416–426. doi: 10.1074/jbc.M703177200. [DOI] [PubMed] [Google Scholar]
- Wu X, Zagranichnaya TK, Gurda GT, Eves EM, Villereal ML. A TRPC1/TRPC3-mediated increase in store-operated calcium entry is required for differentiation of H19-7 hippocampal neuronal cells. J Biol Chem. 2004;279:43392–43402. doi: 10.1074/jbc.M408959200. [DOI] [PubMed] [Google Scholar]
- Yildirim E, Birnbaumer L. TRPC2: molecular biology and functional importance. Handb Exp Pharmacol. 2007:53–75. doi: 10.1007/978-3-540-34891-7_3. [DOI] [PubMed] [Google Scholar]
- Yoshida I, Monji A, Tashiro K, Nakamura K, Inoue R, Kanba S. Depletion of intracellular Ca2+ store itself may be a major factor in thapsigargin-induced ER stress and apoptosis in PC12 cells. Neurochemistry international. 2006;48:696–702. doi: 10.1016/j.neuint.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Yuruker V, Naziroglu M, Senol N. Reduction in traumatic brain injury-induced oxidative stress, apoptosis, and calcium entry in rat hippocampus by melatonin: Possible involvement of TRPM2 channels. Metabolic brain disease. 2015;30:223–231. doi: 10.1007/s11011-014-9623-3. [DOI] [PubMed] [Google Scholar]
- Zhou FW, Matta SG, Zhou FM. Constitutively active TRPC3 channels regulate basal ganglia output neurons. J Neurosci. 2008;28:473–482. doi: 10.1523/JNEUROSCI.3978-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington’s disease. Prog Neurobiol. 2007;81:294–330. doi: 10.1016/j.pneurobio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5:311–322. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]