Table 1.
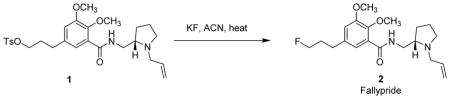
Preparation of fallypride using tracer-level [19F]KF and the comparison with 18F-radiosynthesis
| ||||||
|---|---|---|---|---|---|---|
| Entry | Base | Solvent | T (°C) | Time (min) | Yield (%) | 18F RCY (%)a |
| 1b | K2CO3 (0.8 mg) | ACN | 100 | 30 | n/a | n/a |
| 2 | K2CO3 (3.0 mg) | ACN | 100 | 30 | 41 | 60 |
| 3 | K2CO3 (0.8 mg) | ACN | 100 | 30 | 63 | 74 |
| 4 | TBAB (4.0 mg) | ACN | 100 | 10 | 55 | 68 ± 1.6c |
| 5 | K2CO3 (0.8 mg) | ACN | 100 | 10 | 64 ± 4d | 77 |
| 6 | KHCO3 (0.8 mg) | ACN | 100 | 10 | 55 | 82 |
| 7 | KHCO3 (1.6 mg) | ACN | 100 | 10 | 63 | 79 |
RCY was determined by HPLC analysis of the crude product.
No KF was used to test background reaction. Glass reaction vial was washed with K2CO3. Fallypride concentration of 85 ± 6 ng/mL was detected.
RCY obtained from Ref. 17
Reaction was run in multiple times (n = 4) and found good reproducibility.
