Table 2.
Optimization of MDL100907 synthesis using tracer-level [19F]KFa
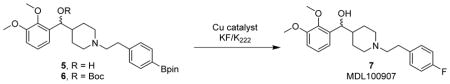
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Precursor (mg) | K2CO3 (mg) | K222 (mg) | Catalystb | Solvent (mL) | T (˚C) | % conv. | |
| R = H | 1 | 5 | 2.8 | 14 | Cu(OTf)2(py)4 | DMF 0.3 | 110 | n/a |
| 2 | 5 | 2.8 | 14 | Cu(OTf)2 | DMF 0.3 | 110 | n/a | |
| 3 | 5 | 0.060 | 0.28 | Cu(OTf)2 | DMF 0.3 | 110 | n/a | |
| 4 | 5 | 0.060 | 0.28 | Cu(OTf)2(py)4 | DMF 0.3 | 110 | n/a | |
|
| ||||||||
| R = Bocc | 5 | 5 | 2.8 | 14 | Cu(OTf)2(py)4 | DMF 0.3 | 110 | n/a |
| 6 | 5 | 0.060 | 0.28 | Cu(OTf)2(py)4 | DMF 0.3 | 110 | 18.1 | |
| 7 | 10 | 0.060 | 0.28 | Cu(OTf)2(py)4 | DMF 0.3 | 110 | 17.0 | |
| 8 | 5 | 0.060 | 0.28 | Cu(OTf)2(py)4 | DMF 0.3 | 120 | 16.9 | |
| 9 | 5 | 0.060 | 0.28 | Cu(OTf)2(py)4 | DMF 0.3 | 100 | 15.5 | |
| 10 | 5 | 0.060 | 0.28 | Cu(OTf)2(py)4 | DMF 0.2 | 110 | 14.8 | |
| 11 | 5 | 0.060 | 0.28 | Cu(OTf)2(py)4d | DMF 0.3 | 110 | 11.3 | |
| 12 | 2.5 | 0.060 | 0.28 | Cu(OTf)2(py)4 | DMF 0.2 | 110 | 8.3 | |
| 13 | 5 | 0.060 | 0.28 | Cu(OTf)2 | DMF 0.3 | 110 | n/a | |
| 14 | 5 | 0.060 | 0.28 | Cu(OTf)2(py)4 | DMF:ACN 1:1 | 110 | n/a | |
In all experiments, 0.15 μg of KF was used as the fluorine source.
Catalyst: precursor = 1:3 (molar ratio)
Deprotection of Boc group was carried out with TFA.
Catalyst: precursor = 1: 2
