Figure 1. Nanoparticle-based IL-12 desensitization therapy protects from PN-induced anaphylaxis.
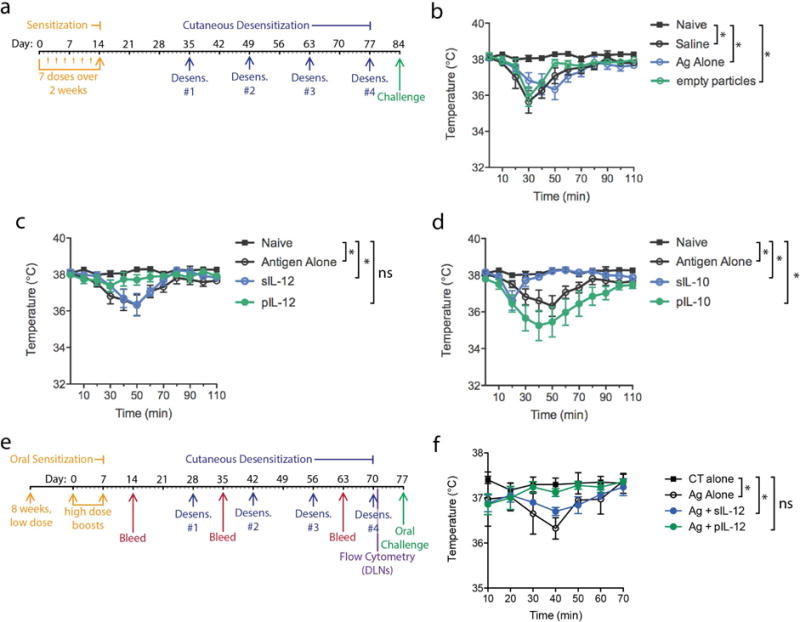
(a) Experimental time course for mouse sensitization to PN, desensitization and PN challenge. For sensitizations, mice were given 7 repeated low dose i.p. injections of PN antigen. Desensitization therapy involved co-administration of 1μg of PN antigen with and without 1ng of soluble or particulate cytokines (IL-12 or IL-10), or treatment with PN antigen alone without any cytokine-containing particles, or treatment with saline alone as control. Mice were challenged by i.p. injection with PN antigen to induce anaphylaxis. Temperatures are shown after challenge for controls and mice desensitized with (b) empty particles, (c) soluble versus particulate IL-12, or (d) soluble versus particulate IL-10. For b–c, Naïve indicates mice that were not PN-sensitized and all other groups were sensitized. pIL-12 and to a lesser extent sIL-10 effectively prevented PN-induced anaphylaxis. * indicates temperatures differ significantly between groups at >1 time point, determined by 2-way ANOVA. (e) Time course of oral sensitization, cutaneous desensitization and oral challenge is presented. For 8 weeks, 50μg of PN antigen was administered by oral gavage weekly. Subsequently, 2 high-dose oral gavage boosts of 1mg PN antigen containing cholera toxin (CT) were given. For some mice, tissues were harvested for immune profiling while others were challenged with 500μg of PN antigen, orally, and temperature was monitored. Control mice were given sensitization doses consisting of CT alone by oral gavage. (f) Anaphylaxis was monitored after oral PN challenge. PN-sensitized mice showed a significant drop in temperature compared to control un-sensitized mice that had received oral CT alone.
