Abstract
Background
Metaplastic breast cancer (MBC), characterized by admixed epithelial, squamous or mesenchymal elements, constitutes <1% of breast cancers and has a poor prognosis but a paradoxically low reported rate of axillary lymph node (LN) involvement. Due to its rarity, data on appropriate axillary management is lacking, prompting this investigation of LN status and outcomes.
Methods
We identified 41 MBC patients treated at our institution 2001–2011 who were followed for a median of 66 months. Statistical analyses evaluated axillary ultrasound (AUS), fine needle aspiration (FNA), and sentinel LN biopsy (SLNB) in association with LN status.
Results
Median tumor size was 2.7 cm and 76% were triple-negative. Twenty-three patients (56%) had preoperative AUS: 9 (39%) showed ≥1 suspicious LN, 6 proceeded to LN FNA and 3 were confirmed positive. Six patients had neoadjuvant chemotherapy, including 2 FNA LN+. Ten patients were LN+ at operation. Among 19 patients undergoing AUS and axillary surgery, AUS ± FNA sensitivity was 100% and specificity was 94%. LN positivity correlated with increasing tumor size, grade and angiolymphatic invasion. 16 patients recurred, 63% with distant disease (10/16) and one with isolated axillary disease after a negative SLNB not preceded by AUS. Overall SLNB accuracy was 96% (23/24), but absent preoperative AUS, 1/7 (14%) of SLNBs were falsely negative.
Conclusions
Our study is the first to specifically address the performance and utility of AUS/FNA and SLNB for MBC patients. AUS/FNA at diagnosis followed by SLN surgery provided accurate nodal staging and critical prognostic information to inform treatment recommendations. We recommend this approach for axillary management of MBC patients.
Keywords: Axillary ultrasound (AUS), metaplastic breast cancer (MBC), outcomes, sentinel lymph node biopsy (SLNB), surgery
Introduction
Metaplastic breast cancer (MBC) is a rare form of invasive breast cancer accounting for <1% of all invasive breast cancers (1-6). The term refers to a heterogeneous group of tumors in which the adenocarcinomatous element is combined with a neoplastic mesenchymal component or other epithelial elements (6,7). The purely epithelial forms include squamous (large cell keratinizing, spindle cell, acantholytic), adenocarcinoma with spindle cell differentiation, and adenosquamous (including mucoepidermoid) (7). Mixed epithelial and mesenchymal types include carcinoma with chondroid metaplasia or osseous metaplasia, or carcinosarcoma (7). Approximately 70% of MBC tumors are said to have a spindle cell component; however, the distribution of histopathologic subtypes vary among the small case series described to date (8,9).
MBCs are reported to present as larger, higher grade, and more frequently estrogen receptor, progesterone receptor and HER2/neu negative (triple-negative) tumors than invasive ductal carcinomas (4,10-13). Further, MBCs respond less frequently to preoperative chemotherapy agents than other triple-negative breast cancers (13,14). Therefore, patients with MBC have been shown to have a significantly worse disease-specific survival than patients with invasive ductal carcinoma of all approximated biologic subtypes including triple-negative tumors (11,13,14).
Paradoxically, patients with MBC have less frequent axillary lymph node (LN) involvement than patients with other forms of invasive breast carcinoma (4,11). Prior reports suggest that the likelihood of LN involvement varies with the histopathologic subtype of MBC such that 10–15% of patients with pure squamous cell carcinoma have LN metastases at presentation while a higher proportion, up to 25%, with chondro-osseous element-containing MBCs are LN-positive (7). Current limited data on surgical management of patients with MBC suggests the majority of patients undergo surgical axillary LN staging; however, data on appropriate preoperative evaluation has not been previously described (4,14).
Due to the rarity of MBC, there is little evidence to guide management of the axilla. Many of the previous studies on MBC include patients diagnosed and treated prior to the widespread availability of breast and axillary ultrasound (AUS) and even prior to adoption of sentinel lymph node biopsy (SLNB) for breast cancer patients. Registry study data lacks granular information on preoperative evaluation and, in past years, on the type of axillary surgery performed. Therefore, to the best of our knowledge, there are no specific studies that address the utility of these management strategies for patients with MBC. This prompted the current study to investigate the role of AUS and SLNB for MBC patients.
Methods
After Institutional Review Board approval, we queried our prospective surgical pathology database to identify all patients with MBC treated at our academic tertiary-care institution between January 2001 and January 2011. Patient, tumor, imaging, treatment, and outcome data were obtained from the electronic medical record. The diagnosis of MBC was confirmed by histopathology slide review.
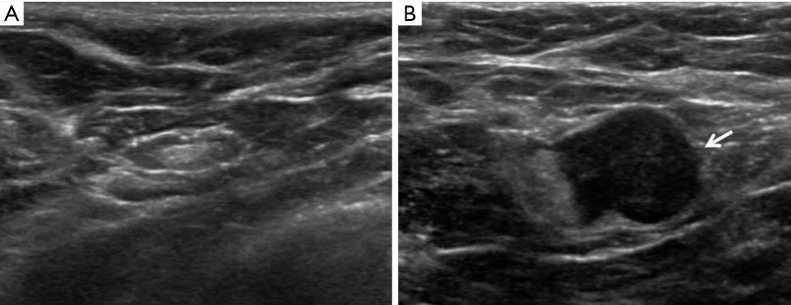
AUS became standard practice for evaluation of our invasive breast cancer patients in 2007. These images, when available, were reviewed by a study radiologist. Sonographic criteria for defining a suspicious axillary LN included cortical thickening >3 mm, eccentric cortical thickening, altered nodal shape, hilar effacement, and/or non-hilar cortical blood flow on color Doppler imaging (Figure 1) (15,16). Patients with sonographically suspicious axillary LNs were recommended for fine needle aspiration (FNA) of the LN with cytology analysis.
Figure 1.
Axillary ultrasound. (A) Normal axillary ultrasound; (B) abnormal axillary ultrasound demonstrates an abnormal axillary lymph node with a markedly thickened cortex, hilar effacement and haziness of the margin (arrow) suggestive of extranodal extension.
Unequal variance t-tests, Chi-square, and Fisher’s exact tests were performed to identify factors associated with LN involvement as appropriate. Performance measures for AUS alone and with FNA were performed using sensitivity and specificity calculations, using the surgical pathology result as the gold standard. These analyses were limited to only patients who underwent AUS and surgical axillary evaluation, and for FNA evaluation were limited to patients with FNA evaluation in addition to AUS; neoadjuvant chemotherapy patients were excluded from these analyses. Kaplan-Meier analyses were performed to evaluate disease-free and breast cancer-specific survival. Statistical significance was set with an alpha of 0.05. Analyses (other than sensitivity and specificity) were performed using JMP Version 10.0.0 (SAS Institute, Cary, NC, USA) software.
Results
Patient and tumor factors
We identified a total of 41 patients who were diagnosed with and treated for MBC over the 11-year study period. Patient demographic, tumor, and treatment features are summarized in Table 1.
Table 1. Patient, tumor and treatment characteristics.
| Characteristics | Patients (n=41) |
|---|---|
| Age (years), median [range] | 60 [33–90] |
| Size (cm), median (IQR) | 2.7 (1.8–5.3) |
| Presentation, n [%] | |
| Asymptomatic | 9 [20] |
| Symptomatic | 33 [80] |
| Grade, n [%] | |
| Low | 7 [17] |
| Intermediate | 6 [15] |
| High | 28 [68] |
| Predominant histology subtype, n [%] | |
| Spindle cell | 19 [46] |
| Mixed | 8 [20] |
| Squamous | 7 [17] |
| Chondroid/osseous | 4 [10] |
| Adenosquamous | 3 [7] |
| Estrogen receptor, n [%] | |
| Positive | 8 [20] |
| Negative | 33 [80] |
| Breast surgery, n [%] | |
| Mastectomy | 26 [63] |
| Wide local excision | 15 [37] |
| Axillary surgery, n [%] | |
| Sentinel lymph node (SLN) | 23 [56] |
| Axillary lymph node dissection (ALND) | 14 [34] |
| SLN followed by ALND | 1 [3] |
| None | 3 [7] |
| Pathologic lymph node status, n [%] | |
| Positive | 10 [26] |
| Negative | 28 [74] |
| Chemotherapy | 22 [54] |
| Adjuvant | 16 [39] |
| Neoadjuvant | 6 [15] |
| None | 19 [46] |
Patients were diagnosed at a median age of 60 years (range, 33–90 years). Median tumor size was 2.7 cm. The majority of patients, 76% (n=31), had triple-negative disease. More patients with LN positive disease presented with a symptomatic finding of a palpable mass or pain versus a mammographically detected abnormality, P=0.03 (Table 2).
Table 2. Comparison of tumor features in lymph node-negative versus lymph node- positive patients.
| Characters | Lymph node negative (n=28) | Lymph node positive (n=10) | P value |
|---|---|---|---|
| Size (cm) | 2.7 (1.6–4.6) | 6.6 (2.5–16.5) | 0.001 |
| Presentation | 0.03 | ||
| Asymptomatic | 7 [100] | 0 [0] | |
| Symptomatic | 21 [68] | 10 [32] | |
| Tumor (T) stage | 0.03 | ||
| T1 | 9 [100] | 0 [0] | |
| T2 | 14 [78] | 4 [22] | |
| T3 | 3 [60] | 2 [40] | |
| T4 | 2 [33] | 4 [67] | |
| Grade | 0.04 | ||
| Low | 6 [100] | 0 [0] | |
| Intermediate/high | 22 [69] | 10 [31] | |
| Estrogen receptor | 0.61 | ||
| Positive (>1%) | 5 [71] | 2 [29] | |
| Negative (<1%) | 23 [74] | 8 [26] | |
| Angiolymphatic invasion | 0.002 | ||
| No | 26 [87] | 4 [13] | |
| Yes | 2 [25] | 6 [75] | |
| Dominant histology | 0.65 | ||
| Adenosquamous | 2 [100] | 0 [0] | |
| Matrix producing | 4 [80] | 1 [20] | |
| Spindle cell | 14 [74] | 5 [26] | |
| Squamous* | 8 [67] | 4 [33] |
Data are shown as median (IQR) or number [percentage]. *, any squamous component.
Over half of the patients (n=23, 56.1%) underwent a preoperative AUS. Of these patients, 39% (n=9) identified at least 1 suspicious axillary LN, of whom 6 had a preoperative FNA LN biopsy, of which 3 were positive for metastasis. Six patients, including the 2 of the 3 patients who were LN positive on FNA, received neoadjuvant chemotherapy.
Performance of AUS with or without fine needle aspiration and sentinel LN surgery
Among 19 patients who had both an AUS, with (n=2) or without (n=2) FNA of suspicious LNs, and axillary surgery, AUS performed with a sensitivity of 100% (2 true positive, 0 false negative) and specificity of 94% (16 true negative, 1 false positive). One patient, who did not undergo a preoperative AUS, suffered a solitary axillary recurrence 8 months after a negative SLNB. Therefore, the accuracy of SLNB was 96% (23/24) overall, but among those without preoperative AUS, 1/7 (14%) SLNBs were falsely negative.
Axillary surgery and pathology findings
Axillary operation consisted of an axillary LN dissection (ALND) in 14 patients, SLNB in 23 patients, SLNB followed by ALND in 1 patient and no axillary surgery in 3 patients. Ten patients were LN positive at operation. LN positivity correlated with increasing tumor size/tumor (T) stage, grade and angiolymphatic invasion (Table 2). While not statistically significant across all histologic subtypes, patients with squamous cell variants of MBC had the highest rate of LN involvement. Six of 41 patients (15%) received neoadjuvant chemotherapy including 2 of the 3 patients pathologically proven to be LN positive after AUS and FNA. Among these patients, 2 had disease progression within the breast, 2 had stable disease and 2 had a partial pathologic response to neoadjuvant chemotherapy. One of these partial responders had biopsy-proven LN positive disease following AUS and FNA at diagnosis and converted to pathologically node negative disease at operation (ALND).
Oncologic outcomes
After a median follow up of 66 months, 16 patients (39%) had developed disease recurrence. The majority of recurrences were as distant disease (10/16, 63%), while 2/16 (12%) were regional only and 2/16 (12%) suffered an in-breast tumor recurrence.
A total of 13 patients (31.7%) succumbed to their disease after a median of 16.7 months (IQR, 10.1–44.5 months). Estimated overall 5-year disease-free survival was 56%. The median breast cancer-specific survival (BCSS) was 20.9 months for LN positive patients, while it was not reached for LN negative patients. Estimated overall 5-year BCSS was greater for LN negative patients at 75%, compared to 12% for LN positive patients, P=0.001 (Figure 2).
Figure 2.
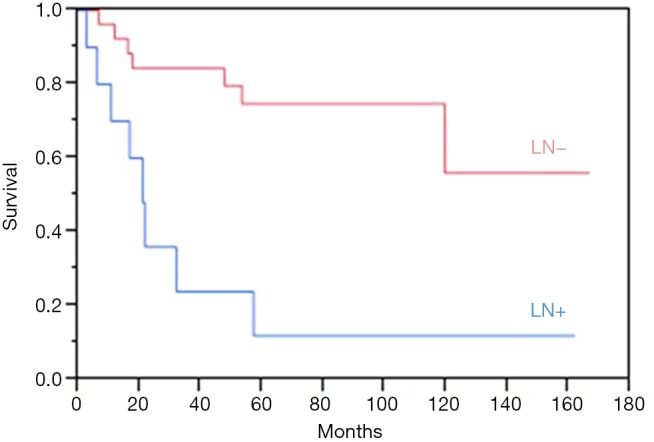
Breast cancer-specific survival for lymph node positive (LN+) versus lymph node negative (LN−) metaplastic breast cancer patients. LN, lymph node.
Discussion
Our study, which evaluated a contemporary cohort of MBC patients, found that AUS with FNA of suspicious LNs performed with perfect sensitivity and excellent specificity. In addition, the accuracy of SLNB for MBC was good. We further found that patients with high grade tumors, larger tumors and tumors exhibiting angiolymphatic invasion were most likely to have LN metastases. These data will help direct treatment recommendations, including axillary management, for patients with MBC.
Treatment guidelines for MBC are similar to those for invasive ductal carcinoma, despite the more aggressive biologic behavior of MBC (17). Our findings are consistent with past studies in that the majority of our patients had hormone-negative disease and approximately 25% had axillary LN involvement (4,10,12,18-20). Despite this lower reported rate of axillary nodal involvement for MBC, the approximately 25% of patients that do have nodal involvement necessitates axillary LN evaluation in all MBC patients for proper staging and treatment (4,14).
The use of preoperative AUS in breast cancer has been shown to have a sensitivity and specificity ranging from 26.4% to 94% and 53% to 98.1%, respectively (15). Its use, in combination with FNA, is well established to help direct management of the axilla in newly diagnosed breast cancer patients (15,16,21). AUS accuracy is influenced both by nodal disease burden and tumor biology (22). While previous reports have shown that AUS may detect LN abnormalities in MBC patients, the utility of AUS in this patient population has not been previously studied (18,19). Our evaluation demonstrated that AUS, with or without FNA of suspicious LNs, performed with a perfect sensitivity and excellent specificity (94%) for MBC patients. Enhanced clinical staging with this approach is useful for formulating treatment recommendations vis-à-vis selection of patients for neoadjuvant chemotherapy and operative management of the axilla.
The use of neoadjuvant chemotherapy for the treatment of triple-negative breast cancers has been increasing in the United States (23). For the treatment of MBC, however, the utility of neoadjuvant chemotherapy is not as clear, as the tumor response varies, and, as we noted in the current study, has not been associated with the substantial pathologic response rates seen with triple-negative invasive ductal breast cancers (13,14). However, we did note that one patient who was pathologically LN positive at diagnosis and had a pathologic partial response to neoadjuvant chemotherapy in the breast did convert to being pathologically node negative at ALND.
The current standard for axillary management of the more common types of clinically node-negative invasive breast cancer includes SLNB, which performs with a false negative rate of just over 7% (15,24). In our MBC patient population, SLNB was 96% accurate, but had a 14% false negative rate with axillary recurrence following a negative SLNB in a solitary patient who did not have a preoperative AUS. It is important to accurately assess the axilla in patients with MBC as, despite having the paradoxically lower rate of LN involvement than with other invasive breast cancers, these patients also have comparatively poorer progression-free and overall survival (11-14,25). We found a substantially diminished BCSS for node-positive versus node-negative MBC patients. Not only does this provide key prognostic information but may identify patients who might be considered for more aggressive and novel multidisciplinary treatment regimens.
Limitations to our study include its retrospective design and modest sample size. Our sample size limited our ability to draw conclusions of the performance of neoadjuvant chemotherapy in this population and false negative rates of SLNB. Additionally, we were unable to assess prognosis based on histopathology subtype. While one prior study showed no significant difference in prognosis based on molecular or histologic subtype of MBC (26), we were unable to confirm this finding. Further evaluation of prognosis based on molecular analysis and histologic outcomes could help direct therapy and should be the focus of future study. Due to its rarity, sample size is an issue for all single center institutions that strive to evaluate MBC. The granular data we were able to collect from our institutional data allowed us to evaluate the sensitivity and specificity of AUS, which is not possible using national databases, and can help direct axillary management in this important subset of breast cancer patients.
Conclusions
For patients with MBC, AUS with FNA of suspicious LNs performed with perfect sensitivity and excellent specificity. With 5.5 years median follow-up, SLNB proved accurate when preceded by AUS. For patients with MBC, we show that AUS with FNA of suspicious LN followed by SLN surgery provided precise staging information of prognostic significance to patients. To facilitate multidisciplinary treatment decisions, we recommend this approach for the axillary management of MBC patients.
Acknowledgements
Salary support for Drs. Habermann and Murphy is provided by The Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
Ethical Statement: As stated in the text, this study was approved by our Institutional Review Board in accordance with standard guidelines.
Footnotes
Conflicts of Interest: This work which was presented in part as a poster at the San Antonio Breast Annual Cancer Symposium, December, 2017 has not been published previously.
References
- 1.Weigelt B, Eberle C, Cowell CF, et al. Metaplastic breast carcinoma: more than a special type. Nat Rev Cancer 2014;14:147-8. 10.1038/nrc3637 [DOI] [PubMed] [Google Scholar]
- 2.Beatty JD, Atwood M, Tickman R, et al. Metaplastic breast cancer: clinical significance. Am J Surg 2006;191:657-64. 10.1016/j.amjsurg.2006.01.038 [DOI] [PubMed] [Google Scholar]
- 3.Luini A, Aguilar M, Gatti G, et al. Metaplastic carcinoma of the breast, an unusual disease with worse prognosis: the experience of the European Institute of Oncology and review of the literature. Breast Cancer Res Treat 2007;101:349-53. 10.1007/s10549-006-9301-1 [DOI] [PubMed] [Google Scholar]
- 4.Pezzi CM, Patel-Parekh L, Cole K, et al. Characteristics and treatment of metaplastic breast cancer: analysis of 892 cases from the National Cancer Data Base. Ann Surg Oncol 2007;14:166-73. 10.1245/s10434-006-9124-7 [DOI] [PubMed] [Google Scholar]
- 5.Rungta S, Kleer CG. Metaplastic carcinomas of the breast: diagnostic challenges and new translational insights. Arch Pathol Lab Med 2012;136:896-900. 10.5858/arpa.2012-0166-CR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz TL, Mogal H, Papageorgiou C, et al. Metaplastic breast cancer: histologic characteristics, prognostic factors and systemic treatment strategies. Exp Hematol Oncol 2013;2:31. 10.1186/2162-3619-2-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tavassoli FA, Devilee P. World Health Organization classification of tumors: tumors of the breast and femal genital organs. Pathology and genetics of tumors of the digestive system. World Health Organization Classification of Tumors. Lyon, France: IARC Press, 2003:37-41. [Google Scholar]
- 8.Cimino-Mathews A, Verma S, Figueroa-Magalhaes MC, et al. A Clinicopathologic Analysis of 45 Patients With Metaplastic Breast Carcinoma. Am J Clin Pathol 2016;145:365-72. 10.1093/ajcp/aqv097 [DOI] [PubMed] [Google Scholar]
- 9.Harris GC, Pinder SE, O'Malley PF. Invasive Carcinoma: Special Types. In: O'Malley FP, Pinder SE, Mulligan AM. editors. Breast Pathology: A Volume in the Foundations in Diagnostic Pathology Series. 2nd ed. Philadelphia, PA: Saunders, 2011. [Google Scholar]
- 10.Lee H, Jung SY, Ro JY, et al. Metaplastic breast cancer: clinicopathological features and its prognosis. J Clin Pathol 2012;65:441-6. 10.1136/jclinpath-2011-200586 [DOI] [PubMed] [Google Scholar]
- 11.Nelson RA, Guye ML, Luu T, et al. Survival outcomes of metaplastic breast cancer patients: results from a US population-based analysis. Ann Surg Oncol 2015;22:24-31. 10.1245/s10434-014-3890-4 [DOI] [PubMed] [Google Scholar]
- 12.Yu JI, Choi DH, Huh SJ, et al. Unique characteristics and failure patterns of metaplastic breast cancer in contrast to invasive ductal carcinoma: a retrospective multicenter case-control study (KROG 13-07). Clin Breast Cancer 2015;15:e105-15. 10.1016/j.clbc.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 13.Nagao T, Kinoshita T, Hojo T, et al. The differences in the histological types of breast cancer and the response to neoadjuvant chemotherapy: the relationship between the outcome and the clinicopathological characteristics. Breast 2012;21:289-95. 10.1016/j.breast.2011.12.011 [DOI] [PubMed] [Google Scholar]
- 14.Aydiner A, Sen F, Tambas M, et al. Metaplastic breast carcinoma versus triple-negative breast cancer: survival and response to treatment. Medicine (Baltimore) 2015;94:e2341. 10.1097/MD.0000000000002341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Hilli Z, Hieken TJ, Boughey JC. Axillary Ultrasound in the Management of the Newly Diagnosed Breast Cancer Patient. Breast J 2015;21:634-41. 10.1111/tbj.12497 [DOI] [PubMed] [Google Scholar]
- 16.Hieken TJ, Trull BC, Boughey JC, et al. Preoperative axillary imaging with percutaneous lymph node biopsy is valuable in the contemporary management of patients with breast cancer. Surgery 2013;154:831-8; discussion 838-40. 10.1016/j.surg.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 17.NCCN Clinical Practice Guidelines in Oncology. 2017.V2. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx
- 18.Choi BB, Shu KS. Metaplastic carcinoma of the breast: multimodality imaging and histopathologic assessment. Acta Radiol 2012;53:5-11. 10.1258/ar.2011.110341 [DOI] [PubMed] [Google Scholar]
- 19.Lai YC, Hsu CY, Chou YH, et al. Sonographic presentations of metaplastic breast cancers. J Chin Med Assoc 2012;75:589-94. 10.1016/j.jcma.2012.08.005 [DOI] [PubMed] [Google Scholar]
- 20.Leddy R, Irshad A, Rumboldt T, et al. Review of metaplastic carcinoma of the breast: imaging findings and pathologic features. J Clin Imaging Sci 2012;2:21. 10.4103/2156-7514.95435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houssami N, Diepstraten SC, Cody HS, 3rd, et al. Clinical utility of ultrasound-needle biopsy for preoperative staging of the axilla in invasive breast cancer. Anticancer Res 2014;34:1087-97. [PubMed] [Google Scholar]
- 22.Dihge L, Grabau DA, Rasmussen RW, et al. The accuracy of preoperative axillary nodal staging in primary breast cancer by ultrasound is modified by nodal metastatic load and tumor biology. Acta Oncol 2016;55:976-82. 10.3109/0284186X.2016.1146826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puig CA, Hoskin TL, Day CN, et al. National trends in the use of neoadjuvant chemotherapy for hormone receptor-negative breast cancer: A National Cancer Data Base Study. Ann Surg Oncol 2017;24:1242-50. 10.1245/s10434-016-5733-y [DOI] [PubMed] [Google Scholar]
- 24.Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis. Cancer 2006;106:4-16. 10.1002/cncr.21568 [DOI] [PubMed] [Google Scholar]
- 25.Bae SY, Lee SK, Koo MY, et al. The prognoses of metaplastic breast cancer patients compared to those of triple-negative breast cancer patients. Breast Cancer Res Treat 2011;126:471-8. 10.1007/s10549-011-1359-8 [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Lv F, Yang Y, et al. Clinicopathological features and prognosis of metaplastic breast carcinoma: experience of a major Chinese cancer center. PLoS One 2015;10:e0131409. 10.1371/journal.pone.0131409 [DOI] [PMC free article] [PubMed] [Google Scholar]