Abstract
As more and more adrenal neoplasms are found incidentally or symptomatically, the need for interventional procedures has being increasing. In recent years these procedures registered continued steady expansion. Interventional radiology of the adrenal glands comprises angiographic and percutaneous procedures. They may be applied both in benign and in malignant pathologies. The present review reports the current status of indications, techniques results and complications of the image-guided procedures.
Keywords: Adrenal gland, interventional radiology, biopsy, adrenal vein sampling (AVS), percutaneous ablation (PA), embolization, review
Introduction
Adrenal incidentaloma represents the result of the widespread use of abdominal imaging techniques [ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI)] (1-3).
In about 4–5% of patients underwent to abdominal imaging, adrenal masses are reported and prevalence increases with patient age (4,5). The incidence of adrenal incidentaloma in autopsy studies is 6% in 87,065 autopsies (3,6). The ideal diagnostic and therapeutic approaches for adrenal incidentalomas are still not clear (7-9). Imaging plays a crucial role in the distinction between adrenal adenomas, which represent the majority of incidentalomas, and malignant adrenal masses, like metastatic carcinoma, pheochromocytoma and adrenocortical carcinoma (ACC) (10,11). Nevertheless, in some cases a final diagnosis may be done only by a percutaneous biopsy.
Interventional radiology of the adrenal glands comprises percutaneous and angiographic procedures, in particular it plays a role both in the diagnostic phase and in the therapeutic one.
As well as biopsies, also the adrenal vein sampling (AVS) represents an important interventional radiologic procedure indicated during the primary aldosteronism (PA).
Moreover, nowadays percutaneous ablations (PAs) are often proposed for the treatment of lesions not eligible for surgery either due to patient’s comorbid disease or to a refusal for surgery. In some cases, these procedures are indicated as curative intent for treatment of functioning and nonfunctioning adenomas.
Trans arterial embolization (TAE) of adrenal arteries may be performed in emergency setting for the treatment of hemorrhages or to reduce vascularization of hypervascularized lesions as palliative intent alone or in combination with PA, or to bridge for surgery.
In all these scenarios, a multidisciplinary approach is mandatory.
The purpose of this review is to analyze indications, techniques, results and safety both for vascular and non-vascular imaging-guided procedures.
Adrenal percutaneous biopsy
Indications
Imaging identifies dimensions, shape, morphology and contrast enhancement characteristics of the lesions (3).
The risk of malignancy increases with increasing size of the mass and decreases with greater lipid content (12-15).
A cut-off size of 4 cm has been accepted to distinguish between benign and malignant lesions: 6% of masses over 4 cm are malignant and the risk of malignancy in masses over 6 cm is 25% (sensitivity 93%, specificity 25% and positive predictive value 25%) (12).
CT and MRI findings are often able to characterize adrenal masses as benign, but they present low specificity in diagnosing malignancy (13).
The role of adrenal percutaneous biopsy to typify malignancy has been controversial (14). Even though this technique was widely employed in the past, the progresses made in imaging modalities together with the burden of complications, have made this diagnostic modality less frequently used nowadays. Moreover, it’s difficult for fine needle aspiration (FNA) biopsy to distinguish between adrenal adenoma and adrenal cortical carcinoma. FNA is recommended by many authors only in cases of suspected metastatic adrenal lesions (8,15). Clinical indications for needle biopsy are the presence of an adrenal mass in an oncologic patient, the staging or the characterization of a primitive lesion beyond that the differentiation of benign from malignant lesions when imaging findings are not resolutive (16). In almost a third of patients, management can be completely altered on the basis of definitive tissue diagnosis (17). When a nonspecific adrenal mass is found in a patient with a history of extra-adrenal malignancy, the probability of malignancy is ranged between 50% and 75% (18-21).
In patients with high suspicion of ACC, adrenal biopsy should be not recommended due to its low diagnostic accuracy (22) and the anatomopathological limit to distinguish between ACC and degenerated adenoma (9,23-25). In some rare cases, adrenal biopsy may avoid unnecessary surgery identifying uncommon pathologies (lymphoma, infection, or hemorrhage) (21-28).
Patient preparation
A careful review of all imaging studies (US, CT and MRI) is required to establish the image guidance to use for the biopsy and to plan the best approach.
Routine pre-procedural laboratory tests include complete blood count (CBC), coagulation studies (PT, PTT, INR) and metabolic panel (CMP). Before to elective biopsy, anticoagulant therapies are stopped following guidelines (26,27).
Approximately 5% of incidentalomas prove to be pheochromocytomas (5); pre-procedural biochemical testing for pheochromocytoma should be recommended (28).
Although the diagnosis of pheochromocytoma is usually made on the basis of clinical history, imaging and laboratory data, in selected cases, a multidisciplinary team, may consider biopsy unavoidable. In these rare circumstances, during the procedure, a careful monitoring and availability of vasodilators and/or prophylactic adrenergic blockade are suggested (29).
Technique
Different imaging guidance techniques [US, CT, CT fluoroscopy (CTF), MRI, PET-CT and fusion modalities] have been used (30).
Adrenal biopsy of left adrenal masses with endoscopic ultrasound-guided (EUS) through a transgastric approach has also been described (17). The endoscopic approach for right adrenal biopsy is less frequently used due to the less visibility, but can be also performed (17,31). Recently, three-dimensional cone-beam CT (3D CBCT), which uses a combination of CBCT and real-time fluoroscopy, has also been described as imaging guidance for biopsy of adrenal glands (32).
In clinical practice, the choice of imaging guidance modality is based on physician preference, availability and anatomy. Each modality presents advantages and disadvantages.
Advantages of US guidance include real time visualization, absence of radiation, low cost and rapid availability. Disadvantages include operator experience, hostile anatomy with interposed bowel or bony structures.
Advantages of CT guidance include high spatial resolution, possibility to identify easily deep structures, and availability of contrast enhanced images if necessary (Figure 1A,B). But, CT uses radiation, is time-consuming, and real-time oblique imaging requires post-processing reconstructions or tilt gantry.
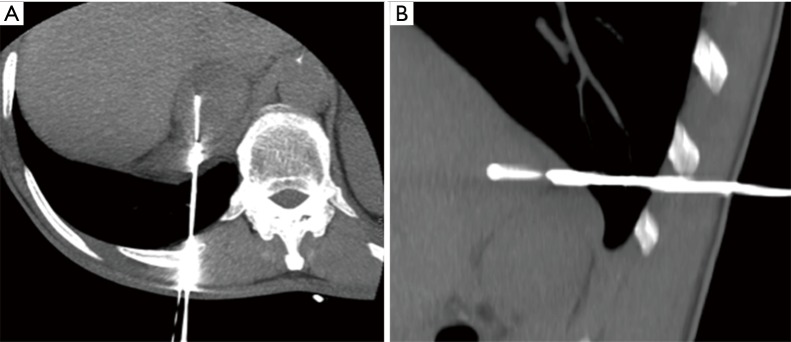
Figure 1.
CT-guided adrenal biopsy: intra-procedural axial (A) and sagittal (B) CT view of a left adrenal gland biopsy.
The association of CT and US is chosen by many operators to get the spatial resolution of CT and the temporal resolution of US (16). The preferred patient position should be the one that makes the patient more comfortable. However, when important structures are in the needle path, the oblique or lateral position may be considered (33).
Trans-pulmonary approach may be a possibility, if lung parenchyma cannot be avoided (34) (Figure 2A,B).
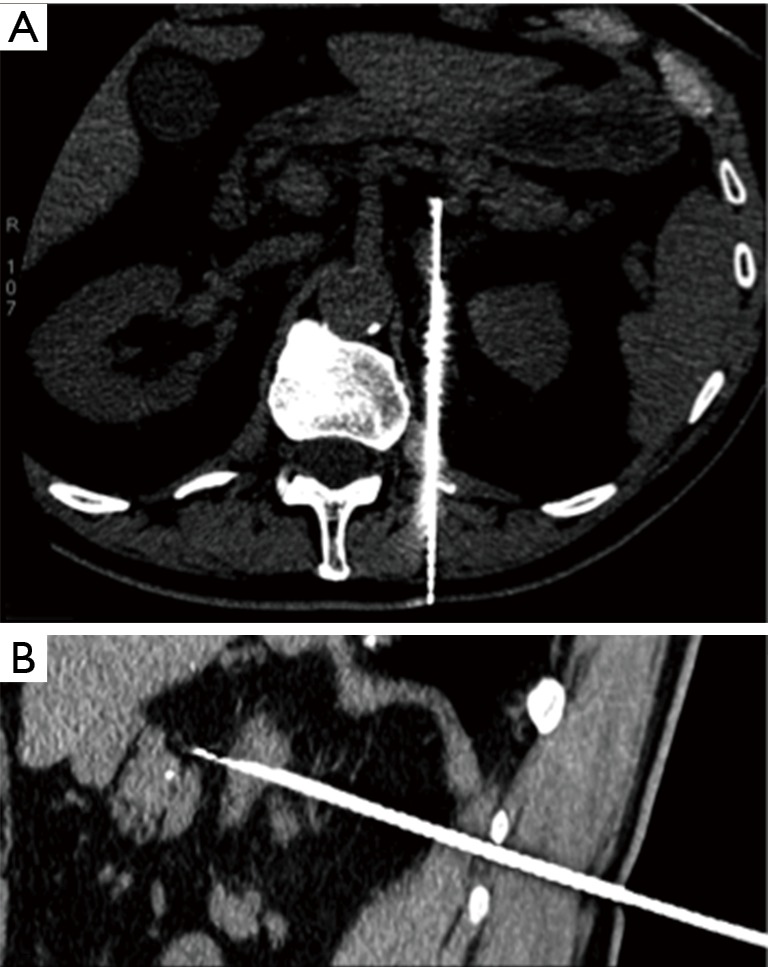
Figure 2.
CT-guided adrenal biopsy: intra-procedural axial (A) and sagittal (B) CT view of a right adrenal gland biopsy performed with a trans-thoracic path.
Additionally, artificial widening of the paravertebral space can be obtained with the hydrodissection technique or with carbon dioxide (CO2) pneumothorax; it consists in the injection under CT guidance of 0.9% saline solution or CO2 respectively (33,35).
A study has recently compared CT-guided adrenal biopsies using posterior approach in patients positioned in the ipsilateral decubitus position and in the prone position, concluding that the first one is less complex but equally safe and as effective as the latter (34).
Jiao et al. (32) have recently published their preliminary experience in adrenal biopsy using 3D CBCT guidance. They used this technique in 54 oncologic patients affected by lesions not easily accessible in plane under CT or ultrasound guidance and reported a technical success rate 100%, with minor complications (3.3%) (32).
Respiratory gating tools may facilitate the procedure, overcoming the challenge for the patient’s inability to cooperate (35).
If FNA is chosen, “capillary pass” technique is suggested to avoid traumatizing the mass and aspiration of blood cells. It consists in moving the needle back and forth within the mass, without syringe aspiration. Only final passes are done with syringe aspiration (16).
Results
The real possibilities of diagnosis of malignancy with adrenal biopsy are unclear. Published studies are heterogeneous (8,24,28).
FNA biopsy is non-diagnostic in 0–28% of the cases (28,36,37); it depends on both the type of biopsied tumor and the experience of the radiologists and biopsy technique (38-40).
Also other factors, related to histology sampling, play a crucial role (41): considering only the diagnostic samples, Bancos et al. reported a sensitivity of 87% and a specificity of 100%. For diagnosing ACC, the sensitivity was 70% and specificity was 98%. For diagnosing metastasis of an extra-adrenal primary malignancy, sensitivity was 87% and specificity was 96% (24). As stated above, the main limitation of adrenal FNA consists in the difficulties to distinguish ACC from benign adrenal tumors. This happens even when the entire specimen is available, but frequently the paucity of tissue doesn’t allow the correct application of the Weiss score system (22). Interestingly, FNA sensitivity varies significantly when considering oncologic (100%) and non-oncologic patients (57%) (42,43), as consequence, adrenal FNA is recommended by many authors only in cases of suspected metastatic adrenal lesions (8,15,44).
Complications
The rate of adrenal biopsy complications varies between different authors. Most studies don’t describe in detail how complications were collected and assessed, and many are retrospective. Other factors are adrenal biopsy technique, characteristics of the lesions, and interventional radiologist’s experience (28). The reported complication rate was between 4% and 13% (38,45). In a recent meta-analysis, comprising of 25 studies (1,339 biopsies), complication rate was 2.5% therefore lower than in the previous data (24). Major complications include hemorrhage (the most frequent), pancreatitis, pneumothorax requiring percutaneous drainage, hemothorax and hypertensive crisis (46). Minor complications include pneumothorax, hematomas, pain, hypertensive episodes, abdominal discomfort, self-limited hypotension and bradycardia (24,46). The most frequent complications reported are hemorrhage and pneumothorax (16). An extremely rare and delayed complication, reported mainly in studies from least recent years, is needle track metastasis seeding (47,48), more frequently encountered with primary ACC (27). Pheochromocytomas and paragangliomas are catecholamines producing tumors. Symptomatology, characterized as ‘‘spells’’, may be caused by uncontrolled secretion of catecholamines. Spells can be precipitated by biopsy, in patients who didn’t undergo pre-procedural biochemical tests to exclude pheochromocytoma (49). Post-biopsy patient monitoring is prudent. Depending on the institutions, the post-procedural observation time varies between 1–4 hours. When biopsies are performed on outpatient setting, if the patient remains asymptomatic and stable, is discharged after instructions about possible late complications and returns to hospital if having any subsequent suspicious symptom (16,33).
AVS
Indications
The AVS consists of taking blood samples directly from the adrenal veins of each side to compare the amount of hormone released by each adrenal gland.
The main indication of AVS is the PA, since the comparison of the aldosterone production between the adrenal glands is essential for the treatment choice (50). Indeed, unilateral over-release of aldosterone is normally associated to aldosterone-producing adenoma (APA), which can be resolved by the adrenalectomy of the affected gland; on the contrary bilateral over-production of aldosterone is generally linked to idiopathic hyper-aldosteronism (IHA), arising from idiopathic hyperplasia of both glands, which requires pharmacological treatment with lifelong mineralocorticoid receptor (MR) blockade (51). In addition, AVS is used to provide lateralization in challenging situations, establishing unilateral aldosterone over release from small CT-MRI-undetectable APAs and excluding hormone hyperproduction in nonfunctioning adrenal mass previously identified with cross-sectional imaging (50).
Conversely, the role of AVS is still controversial in other adrenal hormonal disorders such as Cushing syndrome or pheochromocytomas (52). In the first case, AVS is often useless given the big dimensions of Cortisol Producing Adenomas, which enables an easy detection with cross-sectional imaging; moreover, the stress induced by an interventional investigation can modify the cortisol secretion, impeding a correct lateralization of the disorder (52). In the second case, AVS has been increasingly abandoned due to potential hazards linked to the lack of normative concentration values for adrenaline and noradrenaline in adrenal veins and the poor reliability of lateralization ratios, especially in the milder forms (52).
Patient preparation and technique
Since AVS is an invasive investigation, an accurate patient selection is necessary. According to international guidelines, AVS should be performed only in patients who have an unequivocal biochemical diagnosis of PA and are suitable for surgery (51,53). An optimal preparation of the patient should prevent all factors that can potentially influence the secretion of aldosterone, providing misleading results (50). Namely, antihypertensive drugs and MR antagonists should be withdrawn, if possible, or replaced with calcium-channel blockers (the only agents with a negligible effect on renin secretion) (54). Another potentially confounding factor is hypokalemia, since it may decrease aldosterone secretion, masking a unilateral APA. Thus, correction of hypokalemia with oral or intravenous potassium supplements before AVS is necessary (50). In addition, the guidelines suggest to perform AVS in conditions that imply a lower release of aldosterone, keeping the patient in the supine position for one hour before the procedure and avoiding emotional and pain-related stress, which may rise the secretion of ACTH (55,56).
A prior cross-sectional imaging, especially CT, may be helpful to plan AVS, defining the anatomy of the adrenal veins, namely the right one, which is featured by small dimensions and several anatomical variants (57).
AVS does not require the hospitalization of the patient (50). One of the main caveat of the procedure is the pulsatile secretion and the diurnal fluctuation of aldosterone, that may lead either to false-negative results or artificial gradients between the adrenal glands (58). Thus, some authors suggest to perform AVS in the morning, applying a simultaneous bilateral approach, in order to minimize time-related concentration changes, compared with sequential sampling (58). Another possible solution is the infusion of cosyntropin (a synthetic derivative of ACTH), that provides the additional benefit to highlight the lateralization, increasing the APA’s production of aldosterone and reducing the stress effects (59). In this case, bilateral sampling is no more needed. However, at the moment there is no consensus that endorses sequential AVS with cosyntropin stimulation over unstimulated bilateral AVS (60,61).
The right adrenal vein usually arises from the IVC and runs behind to the right, even if in 23% of patients, the course is to the left, and in 11–38% of patients, runs cranially (62).
The left adrenal vein arises from the anterior surface of the gland and joins the left inferior phrenic vein before draining into the left renal vein (63).
Some anatomical variations have been described: a common trunk between the right adrenal vein and an accessory hepatic vein in 8% of the cases, while in 10% it is multiple. A single case report described direct drainage of the left adrenal vein into the IVC (64); in up to 2% of patients the presence of two left adrenal veins was reported (65).
The procedure may be performed both using single or adjacent dual right femoral vein access sites permitting for sequential or simultaneous AVS, respectively (55). The jugular vein can be used if necessary. Recently, AVS via an antecubital approach was described in 190 patients as safe and feasible (63).
Different catheters can be used for the selective catheterization of the adrenal veins; the choice depends on operator experience, availability, and relevant anatomy (66).
The sampling of the left adrenal vein implies the positioning of the catheter tip just beyond the ostium of the left inferior phrenic vein, comprising all the ipsilateral adrenal tributaries (50). The right AVS is usually more challenging and requires the identification of the right adrenal vein, which must be differentiated from possible accessory hepatic veins (50). The correct position of the catheter should be confirmed before and after the blood uptake by the injection of very small amounts of contrast agents to prevent the adrenal vein rupture (50).
In rare cases where catheterization of the left or right adrenal vein is impossible, AVS may still be achieved with the catheter tip just proximal to the expected location of the respective adrenal vein (66). Injection of contrast is performed gently to prevent extravasation and/or thrombosis with consequent infarction. Daunt (55) describes five angiographic appearances of the adrenal gland and vein.
Cortisol levels obtained at each site (67). The cortisol levels obtained from the adrenal catheters are commonly 10 times the value obtained from the peripheral sheath (59), (but a multiple of at least 2–3 times the peripheral value is adequate to confirm catheter placement). The ratio of plasma cortisol concentrations (PCC) in the considered adrenal vein and in the infra-adrenal IVC or a peripheral vein is defined by the Selectivity Index (SI) (58). The rationale is that cortisol is supposed to be released only from the adrenal cortex and that is not normally hypersecreted by APAs. The SI cut-off values vary according to the technique used for the AVS: it has been suggested the employ of SI values ≥2.0 under unstimulated conditions and ≥3.0 during cosyntropin stimulation (50).
If the success of catheterization is bilaterally confirmed, plasma aldosterone concentrations (PAC) in both adrenal veins should be estimated, in order to determine the lateralization of the hormone production. The guidelines suggest to normalize the PAC with the PCC values to get more reliable results, and to assess the lateralization calculating the Lateralization Index (LI), which represents the ratio of PAC/PCC of the dominant (higher) side over the one of the non-dominant (lower) side (58). Since PA generally arises from benign conditions, which can be also managed with a pharmacological approach, false negatives have just a mild impact on patient conditions. Thus, high LI diagnostic thresholds are usually endorsed in order to avoid false positive-related adrenalectomy. Advisable cut-off values for LI are ≥2.0 under unstimulated conditions and ≥4 during cosyntropin stimulation (68). It must be noticed that the lateralization of aldosterone production can be assessed only if catheterization is bilaterally successful (50).
Unluckily, an incorrect lateralization can be demonstrated only after a surgical treatment, stating persistent hypokalemia or insuppressible PAC associated to low renin levels. Therefore, to prevent useless and risky surgery, all ambiguous results need the repetition of the AVS procedure (51).
Complications
It has been stated that when performed by skilled interventional radiologists, AVS is a safe procedure with a low rate of complications (less than 0.61% of cases according to recent data) (69). Major complications are represented by adrenal vein rupture, thrombosis, dissection, and intra- and periadrenal hematoma (55). In most of cases, all these side effects can be managed with a conservative approach and do not leave long-term consequences. Moreover, when the vascular complications involve the venous drainage of an APA, they can also have a paradox curative outcome (50).
Ablations
Indications of PA and clinical evaluation of the patient
PA includes percutaneous radiofrequency ablation (RFA), cryoablation, microwave (MW), chemical ablation and irreversible electroporation (IRE).
Like in other districts, these techniques are indicated for patients unsuitable for surgery either due to comorbid disease or who refuse surgery (67,70).
PA has been reported to manage all major pathologies affecting adrenal glands: nonfunctioning adenoma; functioning adenoma, including cortisol producing adenoma; aldosteronoma; ACC; pheochromocytoma; and metastasis from renal cell cancer, melanoma, and lung and gastrointestinal tumors (29,71-85).
The final decision for the best treatment must be taken by a multidisciplinary team (MT) involving the interventional radiologist, surgeon, endocrinologist and oncologist (67,70).
Risks and benefits of the PA should be discussed with the patient, who is typically a poor surgical candidate because of the presence of multiple medical and surgical comorbidities.
The major risk of an image-guided procedure is the hemorrhage; all the coagulopathies should be corrected before the PA procedure (INR should be ≤1.5 and the platelet count should be >50,000/µL) (67,86).
For suspected functioning tumors, other laboratory data are usually analyzed, like the serum or urine assays for cortisol, aldosterone and catecholamines (70).
Some papers report preprocedural and periprocedural adrenergic blockade to prevent hypertensive crisis (70,83,87), which could be due of a massive release of catecholamines during the PA of the adrenal lesions (67,73,78,80).
Other complications of adrenal PA include thermal injury to adjacent structures (70,88) and they will be discussed below.
Before the treatment, previous CT and/or MRI should be evaluated to identify and to consider any critical vascular and non-vascular structures that lie adjacent to the adrenal glands (67).
Moreover, if the target lesion is hypervascular, combination therapy with adrenal arterial embolization and PA can be applied to prevent hemorrhagic complication (89).
Although the consensus on the threshold size to treat has not been reached yet, it’s well established that the smaller the maximum lesion diameter, the greater the likelihood of achieving local tumor control (67), in particular the better treatment response has been reported for adrenal mass ≤5 cm (72,76,89).
The need for a pre-procedure biopsy has been discussed. Because no tissue is removed during PA, some authors reported that pre-ablation biopsy should be useful confirming the pathologic diagnosis and it may avoid unnecessary treatment of a benign tumors and an over-estimation of ablation efficacy (90). Nevertheless a review of the literature showed a non-significant necessity of the biopsy before the ablation (70,88) when the diagnosis was established by clinical history and non-invasive imaging, the preprocedural biopsy may be avoided (88). Positive urine catecholamine results obviate biopsy. FDG-PET has been used to differentiate benign from malignant adrenal tumors (91); however, functioning adenomas and pheochromocytomas can present false-positive lesions showing 18F-FDG uptake (92).
In conclusion, factors favoring biopsy are the patients’ preference for a definitive diagnosis, the fact that ablation does not yield a resection specimen and the need to set up follow-up (88). Some authors obtained a pathologic confirmation of the diagnosis with a computed tomography (CT)-guided biopsy performed immediately prior to ablation (76), with results available after completion of the procedure (93).
Patient positioning
Like described for percutaneous adrenal biopsies, most adrenal lesions are accessible via a posterior approach with the patient in the prone position (67). The observations made in the paragraph dedicated to percutaneous biopsies may be considered valid also for PA.
In cases in which there is no direct access to the adrenal glands from a posterior approach because colon or lung obstruct the pathway to the adrenal lesion, transhepatic or transpleural approaches may be used (70).
PA image guidance
The observations made in the paragraph dedicated to percutaneous biopsies may be considered valid also for PA.
MRI guidance has been used as the image guidance of the PA in other organ’s tumors, however there is not consensus yet about its use in adrenal PA (94,95).
Methods of percutaneous adrenal ablation
RFA
RFA consists in delivery of an alternating current, producing ionic agitation, which generates heat (with temperatures of around 50–60 °C) thanks to the Joule effect, and cause coagulative necrosis of the tissue (96).
The use of RFA was described for the treatment of benign and malignant adrenal masses, including metastatic disease. The few published reports hypothesized that results are comparable with those of laparoscopic adrenalectomy (LA); authors focalized on some advantages like the mini-invasive of the procedure with lower morbidity, faster recovery, widespread availability and lower costs (69,70).
Operatively, the procedure is performed inserting within the lesion a needle electrode connected to a generator that produces electromagnetic waves (67) (Figure 3A,B,C,D,E).
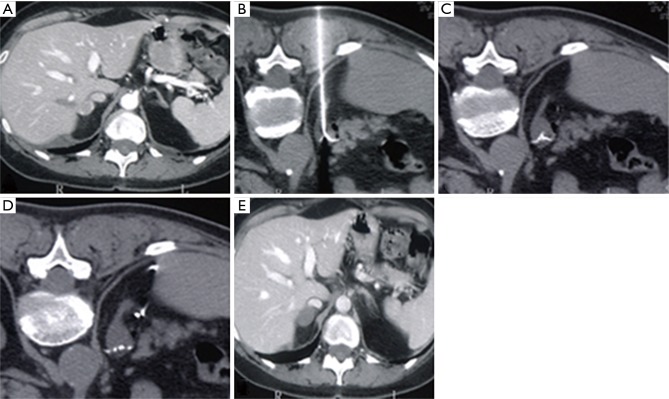
Figure 3.
Percutaneous CT-guided thermal ablation of a right adrenal gland tumor: CT reveals a lesion of the right adrenal gland (A); intra-procedural axial CT views (B-D) confirm the correct position of the needle; CT scan performed during follow up reveals the complete ablation of the lesion (E).
In 2003, Wood et al. reported the first study of imaging-guided RFA of primary and metastatic ACCs (72): the mean diameter was 4.3 cm and follow up (FU) was conducted for a mean of 10.3 months and local control was reached in 8 of 15 lesions. One patient developed an abscess treated with percutaneous drainage and antibiotics. No hypertensive crisis happened.
A second early series reported by Mayo-Smith et al. (76) included 13 tumors (1 pheochromocytoma, 1 aldosteronoma, and 11 metastatic lesions); in a mean FU of 11.2 months successful treatment in 11 of 13 cases was revealed. Even in this study, 12 procedures were performed under conscious sedation and no patients had a hypertensive crisis. The patient with pheochromocytoma was treated for 3 weeks with oral α-blockers and the procedure was monitored by a MT including an anesthesiologist experienced with management of surgical resection of pheochromocytoma.
Carrafiello et al. (71) supported results described by Mayo-Smith et al. (76), indicating that RFA is effective and safe for local control of adrenal metastases (88).
In 2007, Arima et al. treated patients with adrenocortical adenoma and Cushing’s syndrome with RFA (97). Clinical success was defined as improvement in serum cortisol and adrenocorticotropic hormone values and relief of symptoms (mean FU, 33 months) (97).
A report by Venkatesan et al. showed that RFA may be safely performed for metastatic pheochromocytoma given careful attention to peri-procedural management (29). Intra-procedural monitoring and administration of α- and β-adrenergic and catecholamine synthesis inhibition were used. Complete ablation was achieved in 85.7% of cases (mean FU, 12.3 months) and no major complications were observed (29).
Recently, two retrospective studies compared the safety and efficacy of RFA with LA in treating adrenal gland’s APA (98,99). Comparing with LA group, RFA group registered less post-operative pain and shorter operative time (100) less blood loss and lower complication rate was provided with RFA (101).
These considerations lead to the conclusion that imaging-guided percutaneous RFA is effective, safe and it might be a valid alternative for surgery in selected patients with APA.
However, as far as we know, in literature there are not any prospective studies comparing RFA to laparoscopy approach yet.
Microwave ablation (MWA)
MWA uses electromagnetic energy in the microwave range (with frequencies of at least 900 MHz) (102) causing water-molecule agitation in targeted tissue resulting in frictional heat and ultimately cell-death from coagulative necrosis (87).
The advantages of MWA include higher intratumoral temperatures, shorter application times, larger ablation areas and possibly decreased pain during the procedure (87,103-106).
In 2009, Wang et al. performed US-guided MWA on 5 adrenal metastases, with no evidence of recurrence in any of them (mean FU, 19 months) (101).
Two years later, Wolf et al. obtained local control of adrenal metastases in all the 4 patients treated with MWA (mean FU, 14.5 months) (85).
Also, Li et al. reported a study of ten adrenal malignancies, including adrenal metastases, treated with MWA: they obtained local control in all cases (mean FU, 11.3 months) (84). All procedures in that series were performed without general anesthesia. Only one patient experienced hypertensive crisis.
Although some authors suggested that the fast increasing of temperature should induce a hypertensive crisis more frequently than RFA, because of a difference in the setting of excessive catecholamine release (70,84,88), available data suggested that the risk with MWA may be lower, because of the shorter overall time needed to treat adrenal lesions.
Cryoablation
Imaging-guided ablation with cryotherapy consists in cellular destruction by freezing: the cellular damages caused vary including direct (membranes disruption) and indirect lesions (ischemia and coagulative necrosis) (81).
Percutaneous cryoablation systems applied for adrenal tumor use argon gas under high pressure, achieving temperatures from −80 °C to less than −150 °C (88). The alternating cycles of freezing and thawing cause mechanical stress on the cellular membranes up to cell disruption (67). Microvascular thrombosis leads to ischemia and reduce the risk of bleeding (88,100,107).
Depending on the size of the tumor, cryoablation involves the placement from one to 15 probes under imaging guidance to cover the tumor and a 5-mm margin (70).
Monitoring of the area of thermal injury is possible observing the growing ice-ball both with CT and MRI and this enable to avoid damage to adjacent organs (67).
Moreover, cryoablation usually require a smaller dose of sedatives during the procedure because less periprocedural pain compared with RFA (108).
The limitations of cryoablation are the increased risk of hemorrhage caused by microvascular thrombosis and the inability to coagulate tissue during probe withdrawal, such as can be done with RFA or MWA (67,100,107).
Recently, several authors reported the efficacy of the cryoablation in adrenal tumors (87).
In a technical note, Abbas et al. reported 4 patients treated with cryoablation for aldosteronomas (109) with high clinical success.
In 2011, a retrospective study of 12 patients with metastatic tumors to an adrenal gland measuring a mean of 2.7 cm and treated with cryoablation showed successful local control in 11 of 12 patients (mean FU, 18 months) (83). Lysed cells caused a greater release of catecholamines and a consequent hypertensive crisis during the final active thaw phase of cryoablation. The anesthesia team successfully managed all hypertensive crises: the cooperation and coordination with the MT can help ensure a successful outcome (70).
Chemical ablation
Adrenal lesion’s chemical ablation can be performed through imaging-guided percutaneous or trans-ipsilateral artery injection of an agent into the adrenal gland (81). Acetic acid or ethanol has been used. Ethanol works via protein denaturation leading to coagulative necrosis and thrombosis of small vessels (88).
Chemical ablation in the adrenal gland is typically performed using several small (19–22 gauge) needles placed under imaging guidance (67,84,85).
One of the advantages of the chemical ablation is the lower risk to damage adjacent organs. One of the disadvantages is represented by the small volume of necrosis obtained one treatment session, necessitating more frequent treatment sessions than for RFA (67,110).
For tumors <3 cm, ethanol was chosen as the chemical agent, in the other cases, 50% acetic acid solution mixed with iodized oil was chosen because of its greater ability to penetrate septa and to permeate within the tumor (111).
In 2008, Xiao et al. reported the largest cases-series of percutaneous adrenal chemical ablation included 46 lesions (111). In a mean FU of 24 months, in the group of primary adrenal tumors (n=26), a complete response was registered in 92.3% of the cases and a partial response in 7.7%. In the group of adrenal metastases (n=20), a complete response was registered in 30% of the patients and a partial response in 70%. For all responders, tumor volume decreased gradually over the course of 2 years. All patients were treated without general anesthesia. No cases of hypertensive crisis were reported (111).
IRE
IRE is a non-thermal ablation technique that uses short electric pulses to create irreversible pores in the cell membrane. The inability to maintain homeostasis causes apoptosis of the cell population in the target lesion (112). IRE uses needle electrodes placed in or around target lesion to deliver a series of brief direct-current high-voltage pulses (112). These electrical pulses causes muscle fasciculations during the procedure, so IRE requires the use of general anesthesia.
Because IRE destroys tissue without thermal energy, it can be applied close to critical structures with no injury or heat sink and can treat lesions within a sharp, well-defined border (70). This represents an interesting advantage in the treatment of adrenal tumors, however, significant studies about IRE in adrenal tumors treatment have not yet been reported in the literature, but it has been used in the management of liver and pancreatic tumors (112-114).
Thomson et al. (115) investigated the safety of IRE for tumor ablation in 38 patients with 69 tumors unresponsive to conventional treatments. One patient had an unplanned insertion of an electrode tip into the inferior portion of the left adrenal gland during a left upper-pole renal IRE procedure. Although this produced transient hypertension, the gland appeared normal at the CT performed at the end of the procedure. Subsequently, this patient described severe postural hypotension that lasted for 2 months (115).
High intensity focused ultrasound (HIFU)
HIFU is a relatively new technique (116). Cavitation is the result of gas bubbles developing within tissues due to prolonged exposure to high-intensity sonographic energy (116). The bursting of these bubbles within the tissue planes causes cell death bringing transient cavitation (116).
HIFU has been described as a potentially effective nonionizing therapy for a variety of benign and malignant tumours (115,117-123). However, to the best of our knowledge, no cases of the use of HIFU in the management of adrenal tumors have been reported yet (70).
Complications
Some of the possible complications of PA procedure in adrenal gland have already been mentioned, including hypertensive crisis and hemorrhage (124).
Other potential complications include thermal injury to adjacent organs, pneumothorax, infection, and tumor seeding of the ablation probe tract.
Infection is a very rare complication of adrenal ablation procedure and no antibiotics are usually needed before the procedure (70).
The risk of tumor seeding along the probe may be reduced by ablating the probe track during removal (88).
Adrenal insufficiency is extremely rare as more than 90% of adrenal tissue must be destroyed to compromise biochemical adrenal function (76).
All the other considerations reported in the paragraph of percutaneous biopsies may be considered valid for PA.
Adrenal gland embolization
Indications and results
Adrenal artery embolization was used for different aims. Oncologic applications for palliation, such as pain relief, reduction of tumor bulk, and preoperative reduction of tumor vascularity represent a field of application. Emergency embolization for hemostasis of ruptured tumors with retroperitoneal hemorrhage is an additional oncologic application. Adrenal artery embolization has been described for the treatment of hyperaldosteronism, traumatic injuries and adrenal artery aneurysms (125).
Tumor debulking and pain relief
Adrenal artery embolization may be considered as palliative of adrenal neoplasms, treatment of hyperaldosteronism in the setting of aldosteronoma, to bridge for adrenalectomy (126). Adrenal tumors, especially when large, can be painful, and often can be challenging for the surgeon because of the risk of bleeding when highly vascular. There are only few studies concerning experiences of arterial embolization of primary ACCs and adrenal metastatic lesions from renal cell carcinoma and melanoma. O’Keeffe et al. (127) published a report with a series of five patients with adrenal metastases; adrenal embolization resulted in effective palliation of pain; also, tumor volume stabilized or decreased on follow-up CT.
Prabhasavat et al. (128), in a retrospective analysis of three patients stated that the embolization was effective in reducing tumor vascularity and in pain relieving.
Sormaz et al. (129) reported a series of three patients with large hypervascular adrenal tumors who underwent TAE 24 hours before adrenalectomy. Their mayor finding was a marked reduction in hypervascularity and a decrease in the size of collateral vessel and this can be helpful in reducing blood loss during surgery.
Although there are no conclusive data on the usefulness of TAE for HCC adrenal metastasis or for primitive adrenal carcinoma for the purpose of prolonging the overall survival beyond palliation, there are few case reports (124,130,131) that seem to suggest that embolization could have a role in a multidisciplinary approach setting.
The largest series of patients to date is by Yamakado et al. (89) and the aim was to establish the clinical utility of the combined therapy of radiofrequency and arterial chemoembolization in the treatment of patients with adrenal metastasis of hepatocellular carcinoma. They concluded that, even if the cohort of patients was small (n=6), the combination was safe and effective.
Hemorrhagic adrenal mass
Spontaneous adrenal hemorrhage associated with a mass is rare and treatment strategies are not standardized (132), but rupture of adrenal tumors can cause massive retroperitoneal bleeding. Published data are represented by only small series and case reports (133-138) and ideal management has not been clarified (125).
Sepsis, anticoagulation, bleeding disorders, trauma, uncontrolled hypertension, an ongoing α-blocker therapy are all considered risk factors that can lead to spontaneous hemorrhage (125).
Before any intervention, it is important to assess the hemodynamic stability with serial hematocrit levels and cardiac monitoring for hypotension. Also, a biochemical work-up should be performed to rule out a hormonally active tumor. Hemodynamic instability plays an important role in the management of hemorrhagic adrenal masses, especially pheochromocytomas. Shock can be tracked back to either pure hemorrhagic, or due to a massive release of catecholamine associated to the haemorrhage, or to a sudden fall in the blood catecholamine level (139). So, in case of a functional tumor, especially pheochromocytomas, proceeding to surgery could be catastrophic for the risk of life-threatening hypertensive crisis or malignant cardiac arrhythmia; in these cases, endovascular embolization may be the only chance for the patient (132).
Adrenal hormone suppression
Endocrinologically active adrenal tumors treated with embolization include pheochromocytoma, aldosteronoma, adrenal cortical carcinoma, and adrenal adenoma. All of them can produce an excess of adrenal hormones (catecholamines, aldosterone, cortisol) (125).
The first authors to report this procedure were Bunuan et al. in 1978 (140) with the goal to deactivate the tumor and to reduce perioperative complications. In their patients, blood pressure was effectively controlled and the removal of the tumor was facilitated.
Since then, many other case reports have been published (141-144), but, to the best of our knowledge, randomized clinical trial are still lacking.
Pheochromocytomas embolization alone has proved to be insufficient for long-term symptom and hormone control and surgical resection remains the gold standard of treatment, also because approximately 15–17% of pheochromocytomas are discovered to be malignant at the pathological examination (145).
PA is a benign cause of secondary hypertension (10% of the hypertensive population). The cause is an adenoma in 90% (Conn disease) of cases or a nodular hyperplasia in the remnant (146). Surgery is the therapy of choice; however, some patients can benefit from a superselective endovascular approach. Hokotate et al. (143) reported the largest series of 33 cases of aldosteronoma treated with embolization.
The results were encouraging: plasma aldosterone level returned definitively to normal in 82% of the patients after superselective adrenal arterial embolization with high-concentration ethanol.
Adrenal gland hemorrhage
Adrenal gland trauma (AGT) is a rare entity because of the anatomic location in the retroperitoneal cavity protected by others organs (147).
Because of their anatomic distribution, traumatic adrenal hemorrhage is usually associated with multiple other injuries. The adrenal glands are extremely vascularized and an acute hemorrhage from an adrenal trauma may be dangerous (Figure 4A,B,C,D,E).
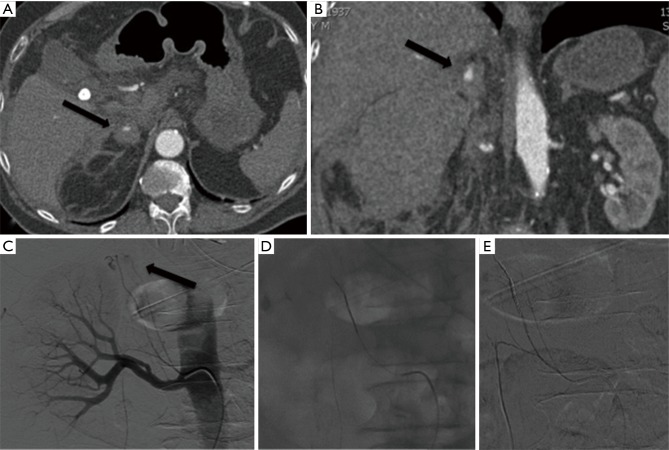
Figure 4.
Active bleeding of the right adrenal gland treated by embolization: axial (A) and coronal (B) view of the CT reveal an active bleeding of the right adrenal gland (black arrow); angiogram confirms contrast medium extravasation (black arrow) from the inferior suprarenal artery (C); its selective catheterization and embolization with microcoils (D); final angiogram reveals complete embolization (E).
The incidence of adrenal injury varies from 0.15% to 4% in various series (148). However, adrenal hemorrhage has been found in 13 of 50 (26%) autopsies of patients who died of severe blunt trauma to the chest or abdomen (149).
Signs and symptoms are non-specific, therefore adrenal hemorrhage in critically ill patients is typically discovered on a CT (148).
Because of the rarity and heterogeneity of the clinical presentation and prognosis of AGT, no guidelines exist.
A recent retrospective review from Liao et al. (150) concluded that extravasation in AGT seen at CECT requires quick treatment. In these cases, adrenal artery embolization could be the modality of choice.
At least four published case reports have described the treatment of isolated adrenal artery hemorrhage after blunt trauma with embolization (151-154). The procedures were performed with microcoils, PVA particles, and N-butyl cyanoacrylate glue. The procedure was effective for all the four patients.
Adrenal artery aneurysms
Visceral arterial aneurysms (VAAs) are a rare entity, often asymptomatic, accidentally diagnosed by CT or MRI. Sometimes, ruptures of VAAs can require emergency treatment (155). In particular, data about adrenal artery aneurysm are limited to only few reports (156-160).
Female gender, pregnancy and portal hypertension represent common risk factors for adrenal artery aneurysms, as for others locations.
Many different embolic agents have been successfully used to treat these conditions and included microcoils with or without PVA particles and fibered platinum coils.
One case of adrenal artery pseudoaneurysm is reported in the literature, successfully embolized with “sandwich” technique (160).
Technical considerations
Anatomical aspects
Prior to adrenal artery embolization is important to understand the complexity of the vascular supply to the adrenal glands and their high variability.
In general, the suprarenal glands are supplied by three main groups of arteries: superior, middle and inferior, which divide into 50 to 60 branches. The superior and the inferior groups are present in all cases; the middle vessels may be missing. The middle one frequently arises from the aorta or the inferior phrenic artery. The superior group generally arises from the ipsilateral inferior phrenic artery. The inferior group are usually branches of the ipsilateral renal artery (161).
Adrenal arteries are of small caliber, can be visualized at catheter aortography in 57–92% of patients without adrenal disease, and can occasionally be seen on abdomen CT acquired with small slice thickness (162).
Because of the complex vascular supply to the adrenal gland by three arteries, embolization of a single artery will not likely result in infarction of the entire gland. If infarction does occur, the presence of an intact contralateral gland will prevent the development of life-threatening adrenal insufficiency (125).
Procedure, embolic agents and complications
The procedure is performed by an arterial vascular access, using proper sheath and catheters (133,134).
The identification of the damaged artery may require sequential angiographic acquisitions. Intra-procedural CBCT imaging associated with the use of vessel-detection software resulted in time-saving, because it facilitates identification of the damaged vessel and its embolization (163).
Each embolic agent has unique characteristics that make it desirable in certain clinical situations and less useful in others; no single embolic agent is suitable for all indications and, to our knowledge, there are no reports in the literature showing superiority of one embolic agent over others (164).
Selection of an embolic agent depends on different factors such as vessel size, the duration of occlusion desired, the need for tissue viability, and the patient’s clinical condition (164).
In literature, the use of coils, polyvinyl alcohol (PVA) particles and gelatin sponge particles are described (133,149,150,161,165).
Liquid embolic agents include ethanol, bucrylate (isobutyl-2-cyanoacrylate), and N-butyl-2-cyanoacrylate has been reported in one case of traumatic adrenal hemorrhage and for hormonal suppression of pheochromocytoma (153,165,166).
Embolization procedures should be performed only by a well-trained and experienced physician. Complications from non-target vessel embolization can be disastrous (134,139), so attention to detail and selection of the proper agent are fundamental.
Patients generally tolerate adrenal artery embolization and post-embolization symptoms can be treated conservatively. The most common treatment complications seen within the first 2 weeks after adrenal artery embolization are back or flank pain, slight fever, labile blood pressure, and modest pleural effusion. Usually, no further treatments are required to control postoperative pain. Persistent hiccups due to diaphragmatic irritation may occur after inferior phrenic artery embolization (127). Adrenal infarction or insufficiency after embolization has not been reported. No immediate deaths or serious complications have been reported (125).
Conclusions
The interventional procedures described are concerned in the management of the primary and secondary conditions of the adrenal glands.
Therapeutic procedures are less invasive, safe alternative or may be considered in association to surgery with high technical and clinical success for palliative adrenal tumor control, suppression of excess adrenal hormone production, and hemostasis of ruptured adrenal tumors, traumatic adrenal injury, and adrenal artery aneurysm occlusion.
New tools, like CBCT can be extremely useful when challenging anatomic conditions occur both in angiographic and percutaneous procedures.
Interventional radiology is a key component of the multidisciplinary team that is required for a successful management of the adrenal gland pathologies.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Davenport C, Liew A, Doherty B, et al. The prevalence of adrenal incidentaloma in routine clinical practice. Endocrine 2011;40:80-3. 10.1007/s12020-011-9445-6 [DOI] [PubMed] [Google Scholar]
- 2.Song JH, Chaudhry FS, Mayo-Smith WW. The incidental adrenal mass on CT: Prevalence of adrenal disease in 1,049 consecutive adrenal masses in patients with no known malignancy. AJR Am J Roentgenol 2008;190:1163-8. 10.2214/AJR.07.2799 [DOI] [PubMed] [Google Scholar]
- 3.Young WF. Clinical practice. The incidentally discovered adrenal mass. N Engl J Med 2007;356:601-10. 10.1056/NEJMcp065470 [DOI] [PubMed] [Google Scholar]
- 4.Bovio S, Cataldi A, Reimondo G, et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest 2006;29:298-302. 10.1007/BF03344099 [DOI] [PubMed] [Google Scholar]
- 5.Young WF. Management approaches to adrenal incidentalomas. A view from Rochester, Minnesota. Endocrinol Metab Clin North Am 2000;29:159-85, x. 10.1016/S0889-8529(05)70122-5 [DOI] [PubMed] [Google Scholar]
- 6.Kloos RT, Gross MD, Francis IR, et al. Incidentally discovered adrenal masses. Endocr Rev 1995;16:460-84. [DOI] [PubMed] [Google Scholar]
- 7.Zeiger MA, Thompson GB, Duh QY, et al. AACE/AAES Guidelines American Association Of Clinical Endocrinologists And American Association Of Endocrine Surgeons Medical Guidelines For The Management Of Adrenal Incidentalomas Writing Committee Endocr Pract 2009;15:1-20. 10.4158/EP.15.S1.1 [DOI] [PubMed] [Google Scholar]
- 8.Terzolo M, Stigliano A, Chiodini I, et al. AME position statement on adrenal incidentaloma. Eur J Endocrinol 2011;164:851-70. 10.1530/EJE-10-1147 [DOI] [PubMed] [Google Scholar]
- 9.Fassnacht M, Arlt W, Bancos I, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol 2016;175:G1-34. 10.1530/EJE-16-0467 [DOI] [PubMed] [Google Scholar]
- 10.Dinnes J, Bancos I, Di Ruffano LF, et al. Management of endocrine disease: Imaging for the diagnosis of malignancy in incidentally discovered adrenal masses: A systematic review and meta-analysis. Eur J Endocrinol 2016;175:R51-64. 10.1530/EJE-16-0461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrera MF, Grant CS, van Heerden JA, et al. Incidentally discovered adrenal tumors: an institutional perspective. Surgery 1991;110:1014-21. [PubMed] [Google Scholar]
- 12.Alexandraki KI, Grossman AB. Adrenal incidentalomas: “The rule of four.” Clin Med (Lond) 2008;8:201-4. 10.7861/clinmedicine.8-2-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cawood TJ, Hunt PJ, O'Shea D, et al. Recommended evaluation of adrenal incidentalomas is costly, has high false-positive rates and confers a risk of fatal cancer that is similar to the risk of the adrenal lesion becoming malignant; time for a rethink? Eur J Endocrinol 2009;161:513-27. 10.1530/EJE-09-0234 [DOI] [PubMed] [Google Scholar]
- 14.Paschou SA, Vryonidou A, Goulis DG, et al. Adrenal incidentalomas: A guide to assessment, treatment and follow-up. Maturitas 2016;92:79-85. 10.1016/j.maturitas.2016.07.017 [DOI] [PubMed] [Google Scholar]
- 15.Nieman LK. Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab 2010;95:4106-13. 10.1210/jc.2010-0457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma KV, Venkatesan AM, Swerdlow D, et al. Image-Guided Adrenal and Renal Biopsy. Tech Vasc Interv Radiol 2010;13:100-9. 10.1053/j.tvir.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeWitt J, Alsatie M, LeBlanc J, et al. Endoscopic ultrasound-guided fine-needle aspiration of left adrenal gland masses. Endoscopy 2007;39:65-71. 10.1055/s-2006-945042 [DOI] [PubMed] [Google Scholar]
- 18.Lenert JT, Barnett CC, Kudelka AP, et al. Evaluation and surgical resection of adrenal masses in patients with a history of extra-adrenal malignancy. Surgery 2001;130:1060-7. 10.1067/msy.2001.118369 [DOI] [PubMed] [Google Scholar]
- 19.Frilling A, Tecklenborg K, Weber F, et al. Importance of adrenal incidentaloma in patients with a history of malignancy. Surgery 2004;136;1289-96. 10.1016/j.surg.2004.06.060 [DOI] [PubMed] [Google Scholar]
- 20.Eloubeidi MA, Black KR, Tamhane A, et al. A large single-center experience of EUS-guided FNA of the left and right adrenal glands: diagnostic utility and impact on patient management. Gastrointest Endosc 2010;71;745-53. 10.1016/j.gie.2009.10.022 [DOI] [PubMed] [Google Scholar]
- 21.Porte HL, Ernst OJ, Delebecq T, et al. Is computed tomography guided biopsy still necessary for the diagnosis of adrenal masses in patients with resectable non-small-cell lung cancer? Eur J Cardiothorac Surg 1999;15;597-601. 10.1016/S1010-7940(99)00047-0 [DOI] [PubMed] [Google Scholar]
- 22.Mazzaglia PJ. Limited Value of Adrenal Biopsy in the Evaluation of Adrenal Neoplasm. Arch Surg 2009;144:465. 10.1001/archsurg.2009.59 [DOI] [PubMed] [Google Scholar]
- 23.Else T, Kim AC, Sabolch A, et al. Adrenocortical carcinoma. Endocr Rev 2014;35:282-326. 10.1210/er.2013-1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bancos I, Tamhane S, Shah M, et al. Diagnosis of endocrine disease: The diagnostic performance of adrenal biopsy: A systematic review and meta-analysis. Eur J Endocrinol 2016;175;R65-80. 10.1530/EJE-16-0297 [DOI] [PubMed] [Google Scholar]
- 25.Fassnacht M, Allolio B. Clinical management of adrenocortical carcinoma. Best Pract Res Clin Endocrinol Metab 2009;23;273-89. 10.1016/j.beem.2008.10.008 [DOI] [PubMed] [Google Scholar]
- 26.O’Donnell MJ, Kearon C, Johnson J, et al. Brief communication: Preoperative anticoagulant activity after bridging low-molecular-weight heparin for temporary interruption of warfarin. Ann Intern Med 2007;146:184-7. 10.7326/0003-4819-146-3-200702060-00007 [DOI] [PubMed] [Google Scholar]
- 27.Zuckerman MJ, Hirota WK, Adler DG, et al. ASGE guideline: The management of low-molecular-weight heparin and nonaspirin antiplatelet agents for endoscopic procedures. Gastrointest Endosc 2005;61:189-94. 10.1016/S0016-5107(04)02392-2 [DOI] [PubMed] [Google Scholar]
- 28.Delivanis DA, Erickson D, Atwell TD, et al. Procedural and clinical outcomes of percutaneous adrenal biopsy in a high-risk population for adrenal malignancy. Clin Endocrinol (Oxf) 2016;85:710-6. 10.1111/cen.13117 [DOI] [PubMed] [Google Scholar]
- 29.Venkatesan AM, Locklin J, Lai EW, et al. Radiofrequency Ablation of Metastatic Pheochromocytoma. J Vasc Interv Radiol 2009;20:1483-90. 10.1016/j.jvir.2009.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta S, Madoff DC. Image-Guided Percutaneous Needle Biopsy in Cancer Diagnosis and Staging. Tech Vasc Interv Radiol 2007;10:88-101. 10.1053/j.tvir.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 31.Martinez M. Role of endoscopic ultrasound fine-needle aspiration evaluating adrenal gland enlargement or mass. World J Nephrol 2014;3:92. 10.5527/wjn.v3.i3.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiao D, Xie N, Wu G, et al. C-arm cone-beam computed tomography with stereotactic needle guidance for percutaneous adrenal biopsy: initial experience. Acta Radiol 2017;58;5:617-24. 10.1177/0284185116661882 [DOI] [PubMed] [Google Scholar]
- 33.Tyng CJ, Bitencourt AG, Martins EB, et al. Technical note: CT-guided paravertebral adrenal biopsy using hydrodissection - A safe and technically easy approach. Br J Radiol 2012;85:e339-42. 10.1259/bjr/16118679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Odisio BC, Tam AL, Avritscher R, et al. CT-guided adrenal biopsy: Comparison of ipsilateral decubitus versus prone patient positioning for biopsy approach. Eur Radiol 2012;22:1233-9. 10.1007/s00330-011-2363-4 [DOI] [PubMed] [Google Scholar]
- 35.Winter TC, Lee FT, Jr, Hinshaw JL. Ultrasound-guided biopsies in the abdomen and pelvis. Ultrasound Q 2008;24:45-68. 10.1097/RUQ.0b013e318168c869 [DOI] [PubMed] [Google Scholar]
- 36.Tirabassi G, Kola B, Ferretti M, et al. Fine-needle aspiration cytology of adrenal masses: A re-assessment with histological confirmation. J Endocrinol Invest 2012;35:590-4. [DOI] [PubMed] [Google Scholar]
- 37.Lumachi F, Borsato S, Tregnaghi A, et al. CT-scan, MRI and image-guided FNA cytology of incidental adrenal masses. Eur J Surg Oncol 2003;29:689-92. 10.1016/S0748-7983(03)00159-8 [DOI] [PubMed] [Google Scholar]
- 38.Quayle FJ, Spitler JA, Pierce RA, et al. Needle biopsy of incidentally discovered adrenal masses is rarely informative and potentially hazardous. Surgery 2007;142:497-502. 10.1016/j.surg.2007.07.013 [DOI] [PubMed] [Google Scholar]
- 39.Lloyd R V. Adrenal cortical tumors, pheochromocytomas and paragangliomas. Mod Pathol 2011;24 Suppl 2:S58-65. 10.1038/modpathol.2010.126 [DOI] [PubMed] [Google Scholar]
- 40.Harisinghani MG, Maher MM, Hahn PF, et al. Predictive value of benign percutaneous adrenal biopsies in oncology patients. Clin Radiol 2002;57:898-901. 10.1053/crad.2002.1054 [DOI] [PubMed] [Google Scholar]
- 41.Saeger W, Fassnacht M, Chita R, et al. High diagnostic accuracy of adrenal core biopsy: Results of the German and Austrian adrenal network multicenter trial in 220 consecutive patients. Hum Pathol 2003;34:180-6. 10.1053/hupa.2003.24 [DOI] [PubMed] [Google Scholar]
- 42.Fassina AS, Borsato S, Fedeli U. Fine needle aspiration cytology (FNAC) of adrenal masses. Cytopathology 2000;11:302-11. 10.1046/j.1365-2303.2000.00261.x [DOI] [PubMed] [Google Scholar]
- 43.Moreira SG, Pow-Sang JM. Evaluation and management of adrenal masses. Cancer Control 2002;9:326-34. 10.1177/107327480200900407 [DOI] [PubMed] [Google Scholar]
- 44.Tsvetov G, Shimon I, Benbassat C. Adrenal incidentaloma: clinical characteristics and comparison between patients with and without extraadrenal malignancy. J Endocrinol Invest 2007;30:647-52. 10.1007/BF03347444 [DOI] [PubMed] [Google Scholar]
- 45.Osman Y, El-Mekresh M, Gomha AM, et al. Percutaneous adrenal biopsy for indeterminate adrenal lesion: Complications and diagnostic accuracy. Urol Int 2010;84:315-8. 10.1159/000288235 [DOI] [PubMed] [Google Scholar]
- 46.Filippiadis DK, Binkert C, Pellerin O, et al. Cirse Quality Assurance Document and Standards for Classification of Complications: The Cirse Classification System. Cardiovasc Intervent Radiol 2017;40:1141-6. 10.1007/s00270-017-1703-4 [DOI] [PubMed] [Google Scholar]
- 47.Habscheid W, Pfeiffer M, Demmrich J, et al. Puncture track metastasis after ultrasound-guided fine-needle puncture biopsy. A rare complication? Dtsch Med Wochenschr 1990;115:212-5. 10.1055/s-2008-1064995 [DOI] [PubMed] [Google Scholar]
- 48.Mody MK, Kazerooni EA, Korobkin M. Percutaneous CT-guided biopsy of adrenal masses: immediate and delayed complications. J Comput Assist Tomogr 1995;19:434-9. 10.1097/00004728-199505000-00017 [DOI] [PubMed] [Google Scholar]
- 49.Vanderveen KA, Thompson SM, Callstrom MR, et al. Biopsy of pheochromocytomas and paragangliomas: Potential for disaster. Surgery 2009;146:1158-66. 10.1016/j.surg.2009.09.013 [DOI] [PubMed] [Google Scholar]
- 50.Rossi GP, Auchus RJ, Brown M, et al. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension 2014;63:151-60. 10.1161/HYPERTENSIONAHA.113.02097 [DOI] [PubMed] [Google Scholar]
- 51.Funder JW, Carey RM, Fardella C, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2008;93:3266-81. 10.1210/jc.2008-0104 [DOI] [PubMed] [Google Scholar]
- 52.Blondin D, Quack I, Haase M, et al. Indication and technical aspects of adrenal blood sampling. Rofo 2015;187:19-28. [DOI] [PubMed] [Google Scholar]
- 53.Nishikawa T, Omura M, Satoh F, et al. Guidelines for the diagnosis and treatment of primary aldosteronism--the Japan Endocrine Society 2009. Endocr J 2011;58:711-21. 10.1507/endocrj.EJ11-0133 [DOI] [PubMed] [Google Scholar]
- 54.Rossi GP. A comprehensive review of the clinical aspects of primary aldosteronism. Nat Rev Endocrinol 2011;7:485-95. 10.1038/nrendo.2011.76 [DOI] [PubMed] [Google Scholar]
- 55.Daunt N. Adrenal Vein Sampling: How to Make It Quick, Easy, and Successful. Radiographics 2005;25 Suppl 1:S143-58. 10.1148/rg.25si055514 [DOI] [PubMed] [Google Scholar]
- 56.Rossi GP. New concepts in adrenal vein sampling for aldosterone in the diagnosis of primary aldosteronism. Curr Hypertens Rep 2007;9:90-7. 10.1007/s11906-007-0017-3 [DOI] [PubMed] [Google Scholar]
- 57.Miotto D, De Toni R, Pitter G, et al. Impact of accessory hepatic veins on adrenal vein sampling for identification of surgically curable primary aldosteronism. Hypertension 2009;54:885-9. 10.1161/HYPERTENSIONAHA.109.134759 [DOI] [PubMed] [Google Scholar]
- 58.Rossi GP, Pitter G, Bernante P, et al. Adrenal vein sampling for primary aldosteronism: the assessment of selectivity and lateralization of aldosterone excess baseline and after adrenocorticotropic hormone;ACTH) stimulation. J Hypertens 2008;26:989-97. 10.1097/HJH.0b013e3282f9e66a [DOI] [PubMed] [Google Scholar]
- 59.Young WF, Stanson AW, Thompson GB, et al. Role for adrenal venous sampling in primary aldosteronism. Surgery 2004;136:1227-35. 10.1016/j.surg.2004.06.051 [DOI] [PubMed] [Google Scholar]
- 60.Seccia TM, Miotto D, De Toni R, et al. Adrenocorticotropic hormone stimulation during adrenal vein sampling for identifying surgically curable subtypes of primary aldosteronism comparison of 3 different protocols. Hypertension 2009;53:761-6. 10.1161/HYPERTENSIONAHA.108.128553 [DOI] [PubMed] [Google Scholar]
- 61.Rossi GP, Ganzaroli C, Miotto D, et al. Dynamic testing with high-dose adrenocorticotrophic hormone does not improve lateralization of aldosterone oversecretion in primary aldosteronism patients. J Hypertens 2006;24:371-9. 10.1097/01.hjh.0000202818.10459.96 [DOI] [PubMed] [Google Scholar]
- 62.Matsuura T, Takase K, Ota H, et al. Radiologic anatomy of the right adrenal vein: Preliminary experience with MDCT. AJR Am J Roentgenol 2008;191:402-8. 10.2214/AJR.07.3338 [DOI] [PubMed] [Google Scholar]
- 63.Jiang X, Dong H, Peng M, et al. A Novel Method of Adrenal Venous Sampling via an Antecubital Approach. Cardiovasc Intervent Radiol 2017;40:388-93. 10.1007/s00270-016-1524-x [DOI] [PubMed] [Google Scholar]
- 64.Stack SP, Rösch J, Cook DM, et al. Anomalous left adrenal venous drainage directly into the inferior vena cava. J Vasc Interv Radiol 2001;12:385-7. 10.1016/S1051-0443(07)61922-8 [DOI] [PubMed] [Google Scholar]
- 65.Parnaby CN, Galbraith N, O’Dwyer PJ. Experience in identifying the venous drainage of the adrenal gland during laparoscopic adrenalectomy. Clin Anat 2008;21:660-5. 10.1002/ca.20706 [DOI] [PubMed] [Google Scholar]
- 66.Khan AS, Hussain HK, Johnson TD, et al. Value of delayed hypointensity and delayed enhancing rim in magnetic resonance imaging diagnosis of small hepatocellular carcinoma in the cirrhotic liver. J Magn Reson Imaging 2010;32:360-6. 10.1002/jmri.22271 [DOI] [PubMed] [Google Scholar]
- 67.Yamakado K. Image-guided ablation of adrenal lesions. Semin Intervent Radiol 2014;31:149-56. 10.1055/s-0034-1373797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mulatero P, Bertello C, Sukor N, et al. Impact of different diagnostic criteria during adrenal vein sampling on reproducibility of subtype diagnosis in patients with primary aldosteronism. Hypertension 2010;55:667-73. 10.1161/HYPERTENSIONAHA.109.146613 [DOI] [PubMed] [Google Scholar]
- 69.Rossi GP, Barisa M, Allolio B, et al. The Adrenal Vein Sampling International Study (AVIS) for identifying the major subtypes of primary aldosteronism. J Clin Endocrinol Metab 2012;97:1606-14. 10.1210/jc.2011-2830 [DOI] [PubMed] [Google Scholar]
- 70.Uppot RN, Gervais DA. Imaging-guided adrenal tumor ablation. AJR Am J Roentgenol 2013;200:1226-33. 10.2214/AJR.12.10328 [DOI] [PubMed] [Google Scholar]
- 71.Carrafiello G, Laganà D, Recaldini C, et al. Imaging-guided percutaneous radiofrequency ablation of adrenal metastases: Preliminary results at a single institution with a single device. Cardiovasc Intervent Radiol 2008;31:762-7. 10.1007/s00270-008-9337-1 [DOI] [PubMed] [Google Scholar]
- 72.Wood BJ, Abraham J, Hvizda JL, et al. Radiofrequency ablation of adrenal tumors and adrenocortical carcinoma metastases. Cancer 2003;97:554-60. 10.1002/cncr.11084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Onik G, Onik C, Medary I, et al. Life-threatening hypertensive crises in two patients undergoing hepatic radiofrequency ablation. AJR Am J Roentgenol 2003;181:495-7. 10.2214/ajr.181.2.1810495 [DOI] [PubMed] [Google Scholar]
- 74.Liang HL, Pan HB, Lee YH, et al. Small functional adrenal cortical adenoma: treatment with CT-guided percutaneous acetic acid injection--report of three cases. Radiology 1999;213:612-5. 10.1148/radiology.213.2.r99nv10612 [DOI] [PubMed] [Google Scholar]
- 75.Maki DD, Haskal ZJ, Matthies A, et al. Percutaneous ethanol ablation of an adrenal tumor. AJR Am J Roentgenol 2000;174:1031-2. 10.2214/ajr.174.4.1741031 [DOI] [PubMed] [Google Scholar]
- 76.Mayo-Smith WW, Dupuy DE. Adrenal Neoplasms: CT-guided Radiofrequency Ablation—Preliminary Results. Radiology 2004;231:225-30. 10.1148/radiol.2311031007 [DOI] [PubMed] [Google Scholar]
- 77.Al-Shaikh AA, Al-Rawas MM, Al-Asnag MA. Primary hyperaldosteronism treated by radiofrequency ablation. Saudi Med J 2004;25:1711-4. [PubMed] [Google Scholar]
- 78.Chini EN, Brown MJ, Farrell MA, et al. Hypertensive crisis in a patient undergoing percutaneous radiofrequency ablation of an adrenal mass under general anesthesia. Anesth Analg 2004;99:1867-9. 10.1213/01.ANE.0000136803.54212.E1 [DOI] [PubMed] [Google Scholar]
- 79.Atwell TD, Wass CT, Charboneau JW, et al. Malignant hypertension during cryoablation of an adrenal gland tumor. J Vasc Interv Radiol 2006;17:573-5. 10.1097/01.RVI.0000197370.83569.33 [DOI] [PubMed] [Google Scholar]
- 80.Lo WK, Vansonnenberg E, Shankar S, et al. Percutaneous CT-guided radiofrequency ablation of symptomatic bilateral adrenal metastases in a single session. J Vasc Interv Radiol 2006;17:175-9. 10.1097/01.RVI.0000188748.51764.CE [DOI] [PubMed] [Google Scholar]
- 81.Beland MD, Mayo-Smith WW. Ablation of adrenal neoplasms. Abdom Imaging 2009;34:588-92. 10.1007/s00261-008-9462-y [DOI] [PubMed] [Google Scholar]
- 82.Tsoumakidou G, Buy X, Zickler P, et al. Life-threatening complication during percutaneous ablation of adrenal gland metastasis: Takotsubo syndrome. Cardiovasc Intervent Radiol 2010;33:646-9. 10.1007/s00270-009-9612-9 [DOI] [PubMed] [Google Scholar]
- 83.Welch BT, Atwell TD, Nichols DA, et al. Percutaneous Image-guided Adrenal Cryoablation: Procedural Considerations and Technical Success. Radiology 2011;258:301-7. 10.1148/radiol.10100631 [DOI] [PubMed] [Google Scholar]
- 84.Li X, Fan W, Zhang L, et al. CT-guided percutaneous microwave ablation of adrenal malignant carcinoma: Preliminary results. Cancer 2011;117:5182-8. 10.1002/cncr.26128 [DOI] [PubMed] [Google Scholar]
- 85.Wolf FJ, Dupuy DE, MacHan JT, et al. Adrenal neoplasms: Effectiveness and safety of CT-guided ablation of 23 tumors in 22 patients. Eur J Radiol 2012;81:1717-23. 10.1016/j.ejrad.2011.04.054 [DOI] [PubMed] [Google Scholar]
- 86.Malloy PC, Grassi CJ, Kundu S, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol 2009;20 Suppl:S240-9. 10.1016/j.jvir.2008.11.027 [DOI] [PubMed] [Google Scholar]
- 87.Ethier MD, Beland MD, Mayo-Smith W. Image-guided ablation of adrenal tumors. Tech Vasc Interv Radiol 2013;16:262-8. 10.1053/j.tvir.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 88.Venkatesan AM, Locklin J, Dupuy DE, et al. Percutaneous Ablation of Adrenal Tumors. Tech Vasc Interv Radiol 2010;13:89-99. 10.1053/j.tvir.2010.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yamakado K, Anai H, Takaki H, et al. Adrenal metastasis from hepatocellular carcinoma: radiofrequency ablation combined with adrenal arterial chemoembolization in six patients. AJR Am J Roentgenol 2009;192:W300-5. 10.2214/AJR.08.1752 [DOI] [PubMed] [Google Scholar]
- 90.Tuncali K, VanSonnenberg E, Shankar S, et al. Evaluation of patients referred for percutaneous ablation of renal tumors: Importance of a preprocedural diagnosis. AJR Am J Roentgenol 2004;183:575-82. 10.2214/ajr.183.3.1830575 [DOI] [PubMed] [Google Scholar]
- 91.Boland GWL, Blake MA, Hahn PF, et al. Incidental adrenal lesions: principles, techniques, and algorithms for imaging characterization. Radiology 2008;249:756-75. 10.1148/radiol.2493070976 [DOI] [PubMed] [Google Scholar]
- 92.Low G, Dhliwayo H, Lomas DJ. Adrenal neoplasms. Clin Radiol 2012;67:988-1000. 10.1016/j.crad.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 93.Zagoria RJ. Imaging-guided radiofrequency ablation of renal masses. Radiographics 2004;24 Suppl 1:S59-71. 10.1148/rg.24si045512 [DOI] [PubMed] [Google Scholar]
- 94.Tatli S, Morrison PR, Tuncali K, et al. Interventional MRI for Oncologic Applications. Tech Vasc Interv Radiol 2007;10:159-70. 10.1053/j.tvir.2007.09.008 [DOI] [PubMed] [Google Scholar]
- 95.Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided Tumor Ablation: Standardization of Terminology and Reporting Criteria for the Society of Interventional Radiology Technology Assessment Committee and the International Working Group on Image-guided Tumor Ablation. Radiology 2005;235:728-39. 10.1148/radiol.2353042205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rhim H, Goldberg SN, Dodd GD 3rd, et al. Essential Techniques for Successful Radio-frequency Thermal Ablation of Malignant Hepatic Tumors. Radiographics 2001;21 Spec No:S17-35; discussion S36-9. [DOI] [PubMed]
- 97.Arima K, Yamakado K, Suzuki R, et al. Image-Guided Radiofrequency Ablation for Adrenocortical Adenoma with Cushing Syndrome: Outcomes After Mean Follow-up of 33 Months. Urology 2007;70:407-11. 10.1016/j.urology.2007.04.032 [DOI] [PubMed] [Google Scholar]
- 98.Yang R, Xu L, Lian H, et al. Retroperitoneoscopic-guided cool-tip radiofrequency ablation of adrenocortical aldosteronoma. J Endourol 2014;28:1208-14. 10.1089/end.2013.0635 [DOI] [PubMed] [Google Scholar]
- 99.Yang MH, Tyan YS, Huang YH, et al. Comparison of radiofrequency ablation versus laparoscopic adrenalectomy for benign aldosterone-producing adenoma. Radiol Med 2016;121;811-9. 10.1007/s11547-016-0662-1 [DOI] [PubMed] [Google Scholar]
- 100.Goel RK, Kaouk JH. Probe ablative treatment for small renal masses: Cryoablation vs. radio frequency ablation. Curr Opin Urol 2008;18:467-73. 10.1097/MOU.0b013e32830a735b [DOI] [PubMed] [Google Scholar]
- 101.Wang Y, Liang P, Yu X, et al. Ultrasound-guided percutaneous microwave ablation of adrenal metastasis: Preliminary results. Int J Hyperthermia 2009;25:455-61. 10.1080/02656730903066608 [DOI] [PubMed] [Google Scholar]
- 102.Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics 2005;25 Suppl 1:S69-83. 10.1148/rg.25si055501 [DOI] [PubMed] [Google Scholar]
- 103.Skinner MG, Iizuka MN, Kolios MC, et al. A theoretical comparison of energy sources--microwave, ultrasound and laser--for interstitial thermal therapy. Phys Med Biol 1998;43:3535-47. 10.1088/0031-9155/43/12/011 [DOI] [PubMed] [Google Scholar]
- 104.Stauffer PR, Rossetto F, Prakash M, et al. Phantom and animal tissues for modelling the electrical properties of human liver. Int J Hyperthermia 2003;19:89-101. 10.1080/0265673021000017064 [DOI] [PubMed] [Google Scholar]
- 105.Wright AS, Lee FT, Mahvi DM. Hepatic Microwave Ablation With Multiple Antennae Results in Synergistically Larger Zones of Coagulation Necrosis. Ann Surg Oncol 2003;10:275-83. 10.1245/ASO.2003.03.045 [DOI] [PubMed] [Google Scholar]
- 106.Shock SA, Meredith K, Warner TF, et al. Microwave ablation with loop antenna: in vivo porcine liver model. Radiology 2004;231:143-9. 10.1148/radiol.2311021342 [DOI] [PubMed] [Google Scholar]
- 107.Weber SM, Lee FT., Jr Expanded treatment of hepatic tumors with radiofrequency ablation and cryoablation. Oncology (Williston Park) 2005;19:27-32. [PubMed] [Google Scholar]
- 108.Allaf ME, Varkarakis IM, Bhayani SB, et al. Pain control requirements for percutaneous ablation of renal tumors: cryoablation versus radiofrequency ablation--initial observations. Radiology 2005;237:366-70. 10.1148/radiol.2371040829 [DOI] [PubMed] [Google Scholar]
- 109.Abbas A, Idriz S, Railton NJ, et al. Image-guided ablation of Conn’s adenomas in the management of primary hyperaldosteronism. Clin Radiol 2013;68:279-83. 10.1016/j.crad.2012.06.137 [DOI] [PubMed] [Google Scholar]
- 110.Livraghi T, Goldberg SN, Lazzaroni S, et al. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology 1999;210:655-61. 10.1148/radiology.210.3.r99fe40655 [DOI] [PubMed] [Google Scholar]
- 111.Xiao YY, Tian JL, Li JK, et al. CT-guided percutaneous chemical ablation of adrenal neoplasms. AJR Am J Roentgenol 2008;190:105-10. 10.2214/AJR.07.2145 [DOI] [PubMed] [Google Scholar]
- 112.Davalos RV, Mir LM, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng 2005;33:223-31. 10.1007/s10439-005-8981-8 [DOI] [PubMed] [Google Scholar]
- 113.Martin RCG, McFarland K, Ellis S, et al. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J Am Coll Surg 2012;215:361-9. 10.1016/j.jamcollsurg.2012.05.021 [DOI] [PubMed] [Google Scholar]
- 114.Kingham TP, Karkar AM, D’Angelica MI, et al. Ablation of perivascular hepatic malignant tumors with irreversible electroporation. J Am Coll Surg 2012;215:379-87. 10.1016/j.jamcollsurg.2012.04.029 [DOI] [PubMed] [Google Scholar]
- 115.Thomson KR, Cheung W, Ellis SJ, et al. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol 2011;22:611-21. 10.1016/j.jvir.2010.12.014 [DOI] [PubMed] [Google Scholar]
- 116.Zhang L, Wang ZB. High-intensity focused ultrasound tumor ablation: Review of ten years of clinical experience. Front Med China 2010;4:294-302. 10.1007/s11684-010-0092-8 [DOI] [PubMed] [Google Scholar]
- 117.Hynynen K, Pomeroy O, Smith DN, et al. MR Imaging-guided Focused Ultrasound Surgery of Fibroadenomas in the Breast: A Feasibility Study. Radiology 2001;219:176-85. 10.1148/radiology.219.1.r01ap02176 [DOI] [PubMed] [Google Scholar]
- 118.Wu F, Wang ZB, Zhu H, et al. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: initial experience. Radiology 2005;236:1034-40. 10.1148/radiol.2362041105 [DOI] [PubMed] [Google Scholar]
- 119.Zhang L, Chen WZ, Liu YJ, et al. Feasibility of magnetic resonance imaging-guided high intensity focused ultrasound therapy for ablating uterine fibroids in patients with bowel lies anterior to uterus. Eur J Radiol 2010;73:396-403. 10.1016/j.ejrad.2008.11.002 [DOI] [PubMed] [Google Scholar]
- 120.Wu F, Wang ZB, Chen WZ, et al. Extracorporeal focused ultrasound surgery for treatment of human solid carcinomas: early Chinese clinical experience. Ultrasound Med Biol 2004;30:245-60. 10.1016/j.ultrasmedbio.2003.10.010 [DOI] [PubMed] [Google Scholar]
- 121.Chen W, Zhu H, Zhang L, et al. Primary Bone Malignancy: Effective Treatment with High-Intensity Focused Ultrasound Ablation. Radiology 2010;255:967-78. 10.1148/radiol.10090374 [DOI] [PubMed] [Google Scholar]
- 122.Li YY, Sha WH, Zhou YJ, et al. Short and long term efficacy of high intensity focused ultrasound therapy for advanced hepatocellular carcinoma. J Gastroenterol Hepatol 2007;22:2148-54. 10.1111/j.1440-1746.2006.04719.x [DOI] [PubMed] [Google Scholar]
- 123.Evans KD, Weiss B, Knopp M. High-Intensity Focused Ultrasound (HIFU) for Specific Therapeutic Treatments: A Literature Review. J Diagnostic Med Sonogr 2007;23:319-27. 10.1177/8756479307307268 [DOI] [Google Scholar]
- 124.Hsieh MH, Lin ZY, Huang CJ, et al. Management of bilateral adrenal metastases from hepatocellular carcinoma: a case report. Kaohsiung J Med Sci 2005;21:371-6. 10.1016/S1607-551X(09)70136-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fowler AM, Burda JF, Kim SK. Adrenal artery embolization: Anatomy, indications, and technical considerations. AJR Am J Roentgenol 2013;201:190-201. 10.2214/AJR.12.9507 [DOI] [PubMed] [Google Scholar]
- 126.Ginat DT, Saad WE, Turba UC. Transcatheter Renal Artery Embolization for Management of Renal and Adrenal Tumors. Tech Vasc Interv Radiol 2010;13:75-88. 10.1053/j.tvir.2010.02.003 [DOI] [PubMed] [Google Scholar]
- 127.O’Keeffe FN, Carrasco CH, Charnsangavej C, et al. Arterial embolization of adrenal tumors: Results in nine cases. AJR Am J Roentgenol 1988;151:819-22. 10.2214/ajr.151.4.819 [DOI] [PubMed] [Google Scholar]
- 128.Prabhasavat K, Ruamcharoenkiat S. Outcomes of Arterial Embolization of Adrenal Tumor in Siriraj Hospital: Case Report. J Med Assoc Thai 2015;98:621-7. [PubMed] [Google Scholar]
- 129.Sormaz IC, Tunca F, Poyanlı A, et al. Preoperative adrenal artery embolization followed by surgical excision of giant hypervascular adrenal masses: report of three cases. Acta Chir Belg 2017:1-7. [Epub ahead of print]. 10.1080/00015458.2017.1312080 [DOI] [PubMed] [Google Scholar]
- 130.Momoi H, Shimahara Y, Terajima H, et al. Management of adrenal metastasis from hepatocellular carcinoma. Surg Today 2002;32:1035-41. 10.1007/s005950200210 [DOI] [PubMed] [Google Scholar]
- 131.Kitagawa Y, Tajika T, Kameoka N, et al. Adrenal metastasis from hepatocellular carcinoma--report of a case. Hepatogastroenterology 1996;43:1383-6. [PubMed] [Google Scholar]
- 132.Marti JL, Millet J, Sosa JA, et al. Spontaneous adrenal hemorrhage with associated masses: Etiology and management in 6 cases and a review of 133 reported cases. World J Surg 2012;36:75-82. 10.1007/s00268-011-1338-6 [DOI] [PubMed] [Google Scholar]
- 133.Habib M, Tarazi I, Batta M. Arterial embolization for ruptured adrenal pheochromocytoma. Curr Oncol 2010;17:65-70. 10.3747/co.v17i6.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ito K, Nagata H, Miyahara M, et al. Embolization for massive retroperitoneal hemorrhage from adrenal pheochromocytoma: A case report. Hinyokika Kiyo 1997;43:571-5. [PubMed] [Google Scholar]
- 135.Pua U, Wong DE. Transarterial embolisation of spontaneous adrenal pheochromocytoma rupture using polyvinyl alcohol particles. Singapore Med J 2008;49:e126-30. [PubMed] [Google Scholar]
- 136.Park JH, Kang KP, Lee SJ, et al. A case of a ruptured pheochromocytoma with an intratumoral aneurysm managed by coil embolization. Endocr J 2003;50:653-6. 10.1507/endocrj.50.653 [DOI] [PubMed] [Google Scholar]
- 137.Hanna JS, Spencer PJ, Savopoulou C, et al. Spontaneous adrenal pheochromocytoma rupture complicated by intraperitoneal hemorrhage and shock. World J Emerg Surg 2011;6:27. 10.1186/1749-7922-6-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kılınç İ, Dumlu EG, Öztürk V, et al. Idiopathic adrenal hematoma mimicking neoplasia: A case report. Int J Surg Case Rep 2016;28:15-7. 10.1016/j.ijscr.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kobayashi T, Iwai A, Takahashi R, et al. Spontaneous rupture of adrenal pheochromocytoma: Review and analysis of prognostic factors. J Surg Oncol 2005;90:31-5. 10.1002/jso.20234 [DOI] [PubMed] [Google Scholar]
- 140.Bunuan HD, Alltree M, Merendino KA. Gel foam embolization of a functioning pheochromocytoma. Am J Surg 1978;136:395-8. 10.1016/0002-9610(78)90304-5 [DOI] [PubMed] [Google Scholar]
- 141.Horton JA, Hrabovsky E, Klingberg WG, et al. Therapeutic embolization of a hyperfunctioning pheochromocytoma. AJR Am J Roentgenol 1983;140:987-8. 10.2214/ajr.140.5.987 [DOI] [PubMed] [Google Scholar]
- 142.O’Halpin D, Legge D, MacErlean DP. Therapeutic arterial embolisation: Report of five years’ experience. Clin Radiol 1984;35:85-93. 10.1016/S0009-9260(84)80001-X [DOI] [PubMed] [Google Scholar]
- 143.Hokotate H, Inoue H, Baba Y, et al. Aldosteronomas: experience with superselective adrenal arterial embolization in 33 cases. Radiology 2003;227:401-6. 10.1148/radiol.2272011798 [DOI] [PubMed] [Google Scholar]
- 144.D’Angelo MW. Transcatheter alcohol embolization of an aldosteronoma. Semin Intervent Radiol 2007;24:96-9. 10.1055/s-2007-971196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jimenez P, Tatsui C, Jessop A, et al. Treatment for Malignant Pheochromocytomas and Paragangliomas: 5 Years of Progress. Curr Oncol Rep 2017;19:83. 10.1007/s11912-017-0643-0 [DOI] [PubMed] [Google Scholar]
- 146.Schirpenbach C, Reincke M. Primary aldosteronism: Current knowledge and controversies in Conn’s syndrome. Nat Clin Pract Endocrinol Metab 2007;3:220-7. 10.1038/ncpendmet0430 [DOI] [PubMed] [Google Scholar]
- 147.Chen KT, Lin TY, Foo NP, et al. Traumatic adrenal haematoma: A condition rarely recognised in the emergency department. Injury 2007;38:584-7. 10.1016/j.injury.2007.01.009 [DOI] [PubMed] [Google Scholar]
- 148.Chernyak V, Patlas MN, Menias CO, et al. Traumatic and non-traumatic adrenal emergencies. Emerg Radiol 2015;22:697-704. 10.1007/s10140-015-1357-y [DOI] [PubMed] [Google Scholar]
- 149.Sevitt S. Post-traumatic adrenal apoplexy. J Clin Pathol 1955;8:185-94. 10.1136/jcp.8.3.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liao CH, Lin KJ, Fu CY, et al. Adrenal gland trauma: Is extravasation an absolute indication for intervention? World J Surg 2015;39:1312-9. 10.1007/s00268-015-2953-4 [DOI] [PubMed] [Google Scholar]
- 151.Tixedor N, Lesnik A, Vernhet H, et al. Embolization treatment of a traumatic adrenal hemorrhage. J Radiol 1999;80:733-5. [PubMed] [Google Scholar]
- 152.Dinc H, Simşek A, Ozyavuz R, et al. Endovascular treatment of massive retroperitoneal haemorrhage due to inferior adrenal artery injury. A case report. Acta Radiol 2002;43:326-8. 10.1034/j.1600-0455.2002.430316.x [DOI] [PubMed] [Google Scholar]
- 153.Kish JW, Katz MD, Marx MV, et al. N-butyl cyanoacrylate embolization for control of acute arterial hemorrhage. J Vasc Interv Radiol 2004;15:689-95. 10.1097/01.RVI.0000133505.84588.8C [DOI] [PubMed] [Google Scholar]
- 154.Ikeda O, Urata J, Araki Y, et al. Acute adrenal hemorrhage after blunt trauma. Abdom Imaging 2007;32:248-52. 10.1007/s00261-006-9046-7 [DOI] [PubMed] [Google Scholar]
- 155.Pitton MB, Dappa E, Jungmann F, et al. Visceral artery aneurysms: Incidence, management, and outcome analysis in a tertiary care center over one decade. Eur Radiol 2015;25:2004-14. 10.1007/s00330-015-3599-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Manners J, Singh R, Page A, et al. Radiological treatment of a spontaneously ruptured inferior adrenal artery aneurysm. Nat Rev Urol 2010;7:694-8. 10.1038/nrurol.2010.184 [DOI] [PubMed] [Google Scholar]
- 157.González Valverde FM, Balsalobre M, Torregrosa N, et al. Spontaneous retroperitoneal hemorrhage from adrenal artery aneurysm. Cardiovasc Intervent Radiol 2007;30:307-9. 10.1007/s00270-006-0029-4 [DOI] [PubMed] [Google Scholar]
- 158.Nakano M, Takada T, Takahashi Y, et al. Spontaneous ruptured adrenal artery aneurysm. J Urol 2003;169:614. 10.1016/S0022-5347(05)63968-7 [DOI] [PubMed] [Google Scholar]
- 159.Birchall D, Carney AS, Morse MH. Case report: Ruptured adrenal artery aneurysm. Clin Radiol 1995;50:732-3. 10.1016/S0009-9260(05)83325-2 [DOI] [PubMed] [Google Scholar]
- 160.Thanos L, Papaioannou G, Grammenou-Pomoni M, et al. Ruptured adrenal artery aneurysm: a case report. Eur Radiol 2000;10:105-7. 10.1007/s003300050013 [DOI] [PubMed] [Google Scholar]
- 161.Manso JC, DiDio LJ. Anatomical variations of the human suprarenal arteries. Ann Anat 2000;182:483-8. 10.1016/S0940-9602(00)80064-3 [DOI] [PubMed] [Google Scholar]
- 162.Toni R, Mosca S, Favero L, et al. Clinical anatomy of the suprarenal arteries: a quantitative approach by aortography. Surg Radiol Anat 1988;10:297-302. 10.1007/BF02107902 [DOI] [PubMed] [Google Scholar]
- 163.Carrafiello G, Ierardi AM, Duka E, et al. Usefulness of Cone-Beam Computed Tomography and Automatic Vessel Detection Software in Emergency Transarterial Embolization. Cardiovasc Intervent Radiol 2016;39:530-7. 10.1007/s00270-015-1213-1 [DOI] [PubMed] [Google Scholar]
- 164.Medsinge A, Zajko A, Orons P, et al. A case-based approach to common embolization agents used in vascular interventional radiology. AJR Am J Roentgenol 2014;203:699-708. 10.2214/AJR.14.12480 [DOI] [PubMed] [Google Scholar]
- 165.Ueno K, Nakajo M, Miyazono N, et al. Transcatheter adrenal arterial embolization of cortisol-producing tumors. Two cases of Cushing’s syndrome. Acta Radiol 1999;40:100-3. [DOI] [PubMed] [Google Scholar]
- 166.Inoue H, Nakajo M, Miyazono N, et al. A case with Cushing’s syndrome treated with arterial ablation of adrenal gland by absolute ethanol. Radiat Med 1993;11:221-3. [PubMed] [Google Scholar]