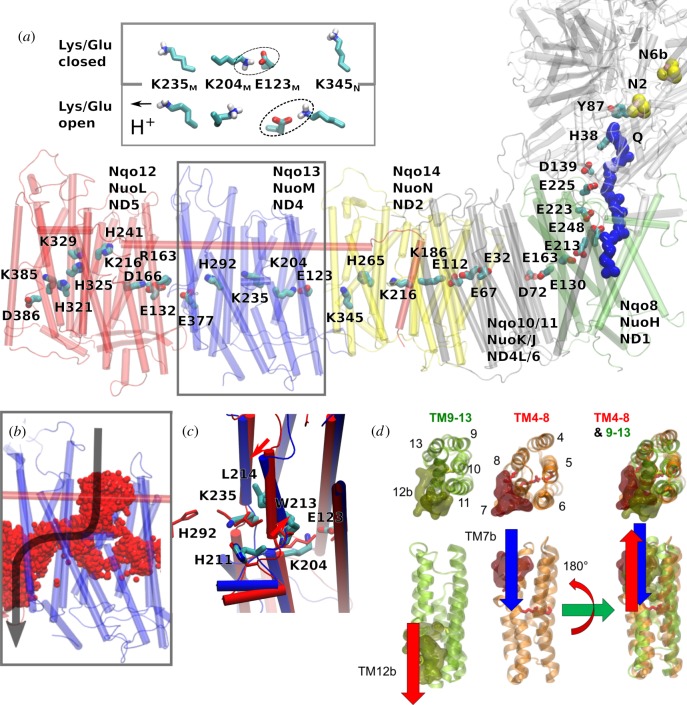
Figure 2.
Structure of the proton-pumping membrane domain in complex I. (a) The membrane domain of complex I showing conserved buried charged/hydrophilic residues. NuoL (red); NuoM (blue); NuoN (yellow); NuoH (green); Q (blue van der Waals representation). Inset: The conformation of the Lys/Glu ion pair (here Lys-204M/Glu-123M) can modulate the pKa of the middle Lys (Lys-235M). (b) Water molecules (in red) establish protonic connectivity in all antiporter-like subunits. The figure shows time-averaged occupancies of water molecules, i.e. water molecules that visit the channel area during the simulation time, in the NuoM subunit based on microsecond MD simulations. (c) Snapshot of structures obtained from MD simulations of open (in blue) and closed (in red) proton channels from the N-side in Nqo13 (NuoM/ND4), showing conformational changes in the broken helix element. (d) The structural symmetry of the antiporter-like subunits with an N-side input channel near broken helix TM7b and output channel near broken helix TM12b. The two five-helical bundles (TM4–8 and TM9-13) are related by rotation and translation symmetry, which is different from the rotation–inversion symmetry found in typical transporters.