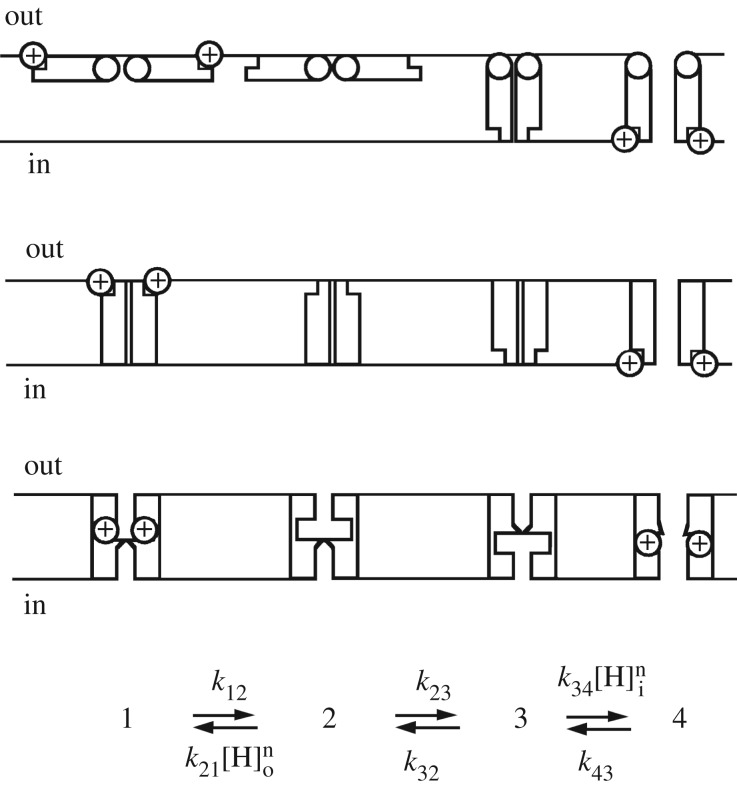
Figure 5.
A four-state ‘butterfly’ model that explains the main features of ΔpH-dependent gating is shown with three possible physical representations. Channel opening occurs from left to right, with state 4 the only conducting state. Top row shows two ‘wings’ that cross the membrane exposing the sites to the opposite solution. The middle row depicts equivalent but distinct internal and external sites, of which only those on one side are accessible. The bottom row shows sites within the pore whose accessibility switches due to a subtle conformational change. Protonation from the external solution stabilizes the deepest closed (non-conducting) configuration (state 1). Deprotonation (state 1 → 2) is required before a conformational change switches the accessibility of the titratable groups to face inwardly (state 2 → 3). Finally, protonation from the inside (state 3 → 4) stabilizes the open channel (state 4). Because no single amino acid substitution abolishes ΔpH dependence [92], multiple groups are probably involved. Reprinted with permission from Cherny et al. [89] (Copyright © 1995 Rockefeller University Press).