Abstract
Cell response to mechanical stimulation was investigated at a subcellular level in protonemal cells of the fern Adiantum capillus-veneris L. by pressing a small part of the cell with a microcapillary. In cells receiving local stimulation, the chloroplasts moved away from the site of stimulation, whereas the nuclei failed to show such avoidance movement. Mechanical stimulation for a period as short as 0.3 min was enough to induce the avoidance response to a maximal level. The avoidance movement of chloroplasts started within 30 min and the plateau level of avoidance was attained around 2 h after stimulation. By tracing the movement of chloroplasts during the response, it was shown that the mobility of chloroplasts near the stimulation site increased transiently within 1 h after the stimulation. After 2 to 3 h, it slowed down to the control level without stimulation. The avoidance response was inhibited by 0.1 mm cytochalasin B and 25 mm 2,3-butanedione monoxime but not by 3.3 μm amiprophosmethyl or 5 mm colchicine. These findings indicate that the protonemal cells were very sensitive to mechanical stimulation and that chloroplasts moved away from the mechanically stimulated site through the actomyosin motile system.
Mechanical stresses such as touch, rain, and wind are important natural factors in the life of a plant because they can physically threaten survival at any time. A number of physiological responses caused by mechanical stimulation have been known for a range of plants since the last century (Darwin, 1882). Common responses to mechanical stimulation are decreased growth and thickening of cell walls, responses that have been termed “thigmomorphogenesis” (Jaffe, 1973). However, studies of these phenomena at the cellular or subcellular level have been limited because only multicellular tissues of higher plants have been used as experimental systems.
To elucidate the mechanisms of mechanoperception and mechanotransduction in plants, it is necessary to know how cells respond to mechanical stimulation at the subcellular level. A few studies of this nature have been performed. Intracellular nuclear migration toward the site of fungal penetration has been studied in dark-grown, suspension-cultured parsley cells (Gross et al., 1993). Nuclear movement toward a site touched with a microneedle was recently reported in epidermal cells of Tradescantia virginiana (Kennard and Cleary, 1997). No other organelles, even chloroplasts, whose movement can be induced readily by light (Nagai, 1993; Wada et al., 1993; Williamson, 1993), have been found to move in response to mechanical stresses in vascular plants.
In the present study, we examined subcellular response to mechanical stimulation in protonemata of a fern, Adiantum capillus-veneris L., which has a simple structure of linearly arranged cells. In these cells, organelles, including chloroplasts, can be readily observed, and chloroplast photomovement has been examined in previous studies (Yatsuhashi et al., 1987a, 1987b; Yatsuhashi and Wada, 1990; Kadota and Wada, 1992; Kagawa and Wada, 1994). Furthermore, mechanical stimulation can be applied to part of an intact cell using a micromanipulator. The site of contact with a needle can be seen under the microscope as the perception site of stimulation. Thus, this type of cell appeared to be ideal for the investigation of the mechanoresponses at the subcellular level. Using this system, we found that chloroplasts moved away from the site of mechanical stimulation induced by pressing with a microcapillary. The details of this phenomenon are reported.
MATERIALS AND METHODS
Plant Materials
Spores of the fern Adiantum capillus-veneris L. were collected in a greenhouse at Tokyo Metropolitan University in 1993 and stored at about 4°C until use. Spores were sterilized for 7 to 8 min with a 0.5% (v/v) bleach solution (Wako Pure Chemical Industries, Osaka) containing 0.1% (w/v) Triton X-100 (Wako), and washed three times with sterile distilled water. The sterilized spores were sown in a line between two layers of agar-gelatin film on a coverslip. The film was made from 0.5% (w/v) Bacto agar (Difco Laboratories, Detroit, MI) and 0.05% (w/v) gelatin (Koso Chemical, Tokyo). Spores were cultured under continuous red light of about 0.5 W m−2 for 9 d in 0.1×-strength, modified Murashige and Skoog mineral salt solution (MS solution; Wada and Furuya, 1970). The resultant protonemata were irradiated with white light of about 4.5 W m−2 for 6 h and then kept in the dark for 2 d. During the dark period, cell division occurred in the apical region of each protonema, giving rise to a short apical cell and a long basal cell. The basal cells of the protonemata were used for the present study. All cultural and experimental procedures were conducted at 25°C ± 1°C.
Light Sources
Fluorescent lamps (FL40SD or FL10D, Toshiba Lighting and Technology, Tokyo) were used as the source of white light. Red light was obtained from the same lamp through a red plastic filter (Shinkolite A no. 102, Mitsubishi Rayon, Tokyo).
Mechanical Stimulation
Protonemata were kept on the coverslip on which they were cultured, placed in a hand-made chamber constructed from a glass-bottomed dish, and anchored with 1% (w/v) agarose VII (Sigma). This chamber was filled with MS solution and placed on the stage of an inverted microscope (Axiovert 10, Zeiss) equipped with a long-distance condenser. Mechanical stimulation of individual cells was performed under the microscope using a microcapillary connected to a joystick-controlled micromanipulator (MO-202, Narishige, Tokyo). The microcapillary was prepared from a 1.0-mm-diameter borosilicate glass tube (GD-1, Narishige) using a vertical two-step puller (PP-84, Narishige). The microcapillary was filled with MS solution. Each cell was pressed with the capillary until deformation of the cell was observed under the microscope. The apical 100- to 300-μm region of the basal cell was used for this study.
Time-Lapse Video Observation
Movement of chloroplasts was monitored under the microscope with IR light obtained through an IR-transmitting filter (IR85, Hoya, Akishima, Japan). The microscope was equipped with an IR-sensitive video camera (C2400–07ER, Hamamatsu Photonics, Hamamatsu, Japan) coupled to a time-lapse video recorder (AG-6730, Panasonic), a video monitor (PVM-14420, Sony), and a video copy processor (SCT-P66, Mitsubishi Electric, Tokyo). For observations, a long-working-distance objective (Achroplan, ×40 LD, NA 0.60, Zeiss) was used.
Quantification of the Chloroplast Avoidance Response
To analyze mechanically induced chloroplast relocation quantitatively, the avoidance response was determined as the percentage of chloroplasts that had moved away from the stimulus region. The number of chloroplasts at the stimulus site before and after mechanical stimulation was counted for each cell. The following equation was used:
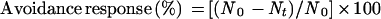 |
where N0 and Nt are the numbers of chloroplasts before and t hours after mechanical stimulation, respectively, within 50 μm in length of the cell (i.e. 25 μm in both the apical and basal directions from the stimulation point).
Inhibitor Treatment
Cytochalasin B (Sigma) was employed as a microfilament-depolymerizing agent. Amiprophosmethyl (APM) and colchicine (both from Sigma) were used to disrupt microtubules. 2,3-Butanedione monoxime (BDM; Sigma), known as an inhibitor of myosin ATPase, was also applied.
Cytochalasin B and APM were dissolved in DMSO as stock solutions of 10 mg mL−1 and 200 μg mL−1, respectively. Final concentrations of cytochalasin B and APM were 50 μg mL−1 (0.1 mm) and 1 μg mL−1 (approximately 3.3 μm), respectively. For treatment with BDM, culture medium containing 25 mm BDM was prepared from a 5 m stock solution in DMSO. The final concentration of DMSO was 0.5% (v/v) in all three cases. Colchicine was dissolved in deionized-distilled water as a stock solution at the concentration of 500 mm. Before use, the stock solution was diluted with the culture medium to 5 mm. To help drugs gain access into the cells, a 0.2% (w/v) solution of the detergent Pluronic F-127 (Sigma) was added in MS solution. Cells were incubated with each drug solution for 2 h before mechanical stimulation. After that, mechanical stimulation was applied for 1 min and the avoidance response was assessed 2 h after the stimulation.
Nucleic Acid Staining
Nucleic acid staining was carried out with SYTO 11 (Molecular Probes). Cells were incubated with 5 μm SYTO 11 in Murashige and Skoog solution for 1 h.
Fluorescence Microscopy
Samples were mounted on a coverslip 0.06 to 0.08 mm thick (no. 00, Matsunami Glass, Osaka). The specimens were observed under a confocal laser scanning inverted microscope (LSM 410, Zeiss). The excitation wavelength for SYTO 11 was 488 nm. The beam splitter used was FT 488/543. A barrier filter, BP 515–525, and an additional beam splitter, FT560, were used for observation. Chlorophyll fluorescence after excitation with either the 488- or 543-nm laser line was obtained using OG 665 as a barrier filter. The pinhole size was 20. Serial optical sections were obtained every 0.75 μm in the z axis. Images were acquired through a ×63 objective (NA 1.4; Plan-Apochromat, Zeiss) at a maximum resolution of 512 × 512 pixels. Every optical section was gained after averaging four scans.
RESULTS
Avoidance Response of Chloroplasts Induced by Mechanical Stimulation
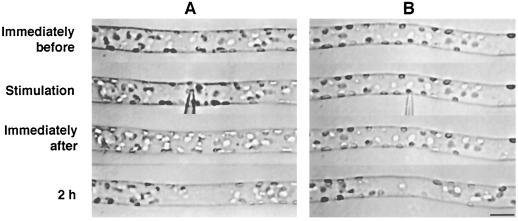
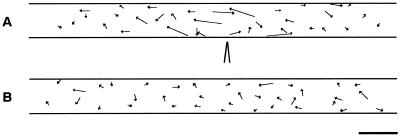
Chloroplasts are known to be distributed randomly throughout the periphery of the long cylindrical basal cell of two-celled A. capillus-veneris protonema when cells are kept in the dark for 2 d, but relocate to the site of optimal light condition for photosynthesis when irradiated (Yatsuhashi et al., 1987b). Therefore, in this study, experiments were carried out in the dark to eliminate the effects of light on chloroplast relocation. When mechanical stimulation was applied to part of the basal cell using a microcapillary, chloroplasts around the stimulated area moved away. Whenever mechanical stimulation was applied to the cell either from above with the flank of the capillary (Fig. 1A) or from the side with the tip of the capillary (Fig. 1B), avoidance responses were observed within 2 h after the onset of mechanical stimulation. The distribution of chloroplasts immediately after the stimulation was not significantly different from that before the stimulation. Subsequently, as a result of chloroplast movement to avoid the stimulated area, a clear zone with fewer chloroplasts compared with the adjacent areas appeared. This mechanically induced chloroplast relocation was also observed in protonemata of Dryopteris filix-mas, Onoclea sensibilis, and Matteucia struthiopteris (data not shown). Mechanical stimulation in the subsequent studies was applied from above with the flank of capillary to avoid cell penetration with the tip.
Figure 1.
Avoidance response of chloroplasts induced by mechanical stimulation. A protonemal cell was stimulated either vertically from above with the flank of a microcapillary (A) or horizontally from the side with the tip of the capillary (B). Note the deformation of the protonemal cell at the stimulation site in A. The protonemal tip was on the right. Bar = 20 μm.
Relationship between Stimulation Periods and the Avoidance Response of Chloroplasts
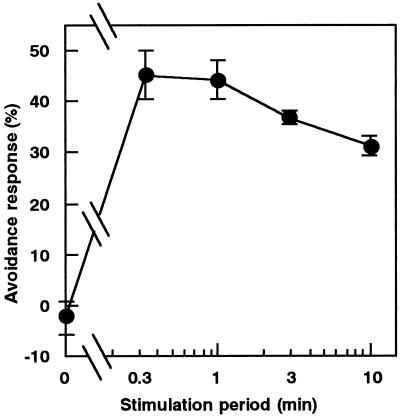
The relationship between the duration of mechanical stimulation and the degree of avoidance response of chloroplasts was investigated. Cells were stimulated for various periods of time with a microcapillary and the avoidance response was quantified after 2 h. The results in Figure 2 show that a maximal avoidance response was obtained even with 0.3 min, the shortest period examined. A stimulation period of 1 min was used in subsequent experiments.
Figure 2.
Relationship between stimulation periods and the chloroplast avoidance response. Protonemata were pushed for various periods from above with a microcapillary. The avoidance response was assessed 2 h after the stimulation. See Methods for quantification of the response. Each point represents the mean ± se obtained from five to 10 protonemata.
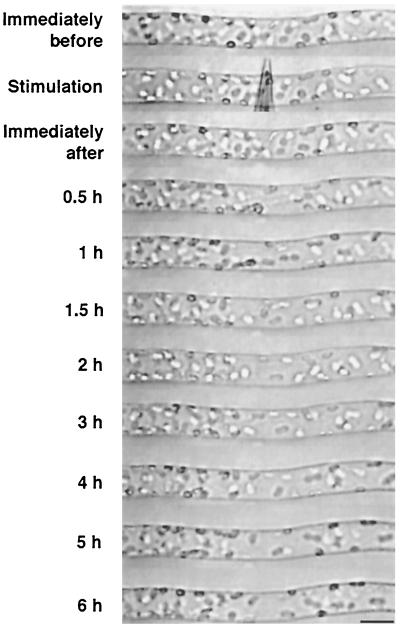
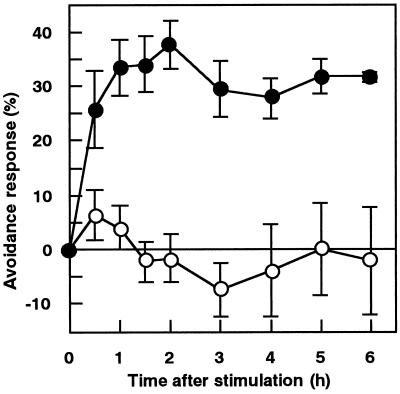
Protonemal cells were mechanically stimulated for 1 min, and chloroplast distribution was continuously observed under the microscope with IR light. Time-course images of a cell during the chloroplast avoidance response are shown in Figure 3. It was found that a clear zone became apparent after 0.5 h and the zone spread in both the apical and basal direction until 2 h after mechanical stimulation. The time course for the avoidance response, shown in Figure 4, revealed that the response reached the maximum level at around 1 to 2 h and remained at a relatively constant level thereafter.
Figure 3.
Time-course images of a cell during the chloroplast avoidance response. Part of a cell was stimulated with a microcapillary for 1 min. The cell was continuously observed under IR light through the IR-sensitive video system. The protonemal tip was on the right. Bar = 20 μm.
Figure 4.
Time course of the chloroplast avoidance response induced by 1 min of mechanical stimulation. Protonemata were pushed for 1 min with a microcapillary from above (•). Control results without mechanical stimulation are also presented (○). Each point represents the mean ± se obtained from four to five protonemata.
Effects of Cytoskeletal Inhibitors
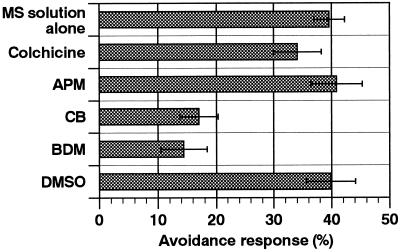
We examined the effects of cytoskeletal inhibitors on the avoidance response of chloroplasts to determine the motile system responsible. After a 2-h pre-incubation in an inhibitor solution, cells were mechanically stimulated and the avoidance responses were observed after a further 2 h in the presence of the inhibitor. The cytochalasin B concentration used is known to disrupt the microfilament architecture in A. capillus-veneris protonemata within 2 h (Kadota and Wada, 1995), and the concentrations of APM and colchicine we used were also sufficient to disturb the microtubule organization within 2 h (Murata and Wada, 1989). As shown in Figure 5, avoidance responses were inhibited by 0.1 mm cytochalasin B and 25 mm BDM but not by 3.3 μm APM or 5 mm colchicine. These results suggest that the response of chloroplasts after mechanical stimulation was mediated by the actomyosin motile system.
Figure 5.
Effects of cytoskeletal inhibitors on the chloroplast avoidance response. Cells were treated with 5 mm colchicine, 3.3 μm APM, 0.1 mm cytochalasin B, or 25 mm BDM in MS solution containing 0.2% detergent. APM, cytochalasin B, and BDM were dissolved with 0.5% DMSO. The control result with 0.5% DMSO alone is also presented. Each bar represents the mean ± se obtained from 11 to 17 protonemata.
Behavior of Each Chloroplast during Avoidance Response
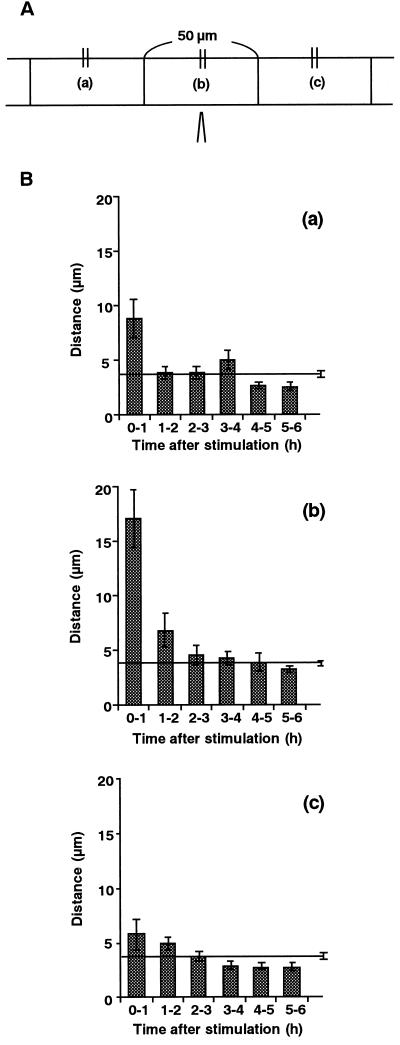
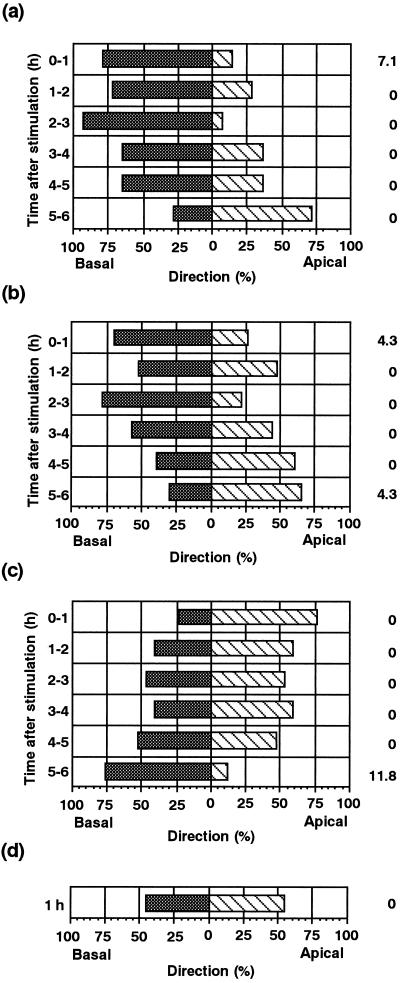
Chloroplasts were tracked individually to analyze the movement of chloroplasts during the avoidance response. The paths of movement of chloroplasts in a single cell for 1 h after stimulation are shown in Figure 6. Chloroplasts in an unstimulated cell moved randomly at a relatively constant velocity. On the other hand, in the stimulated cell, it is clear that the movement of chloroplasts near the stimulated site was activated in an axial direction, while that of chloroplasts far from the site remained at the unstimulated level. Time courses for chloroplast mobility in the regions around the stimulus point were quantitatively examined over intervals of 1 h, as shown in Figure 7. The mobility of chloroplasts in the stimulus region was greatest during the first hour after the stimulation, and gradually slowed down to the control level 1 to 3 h after the stimulation. In both the apical and basal regions, the mobility of the chloroplasts also increased transiently during the first hour, although to a lesser extent than in the area of stimulation. Figure 8 shows the direction of chloroplast movement at hourly intervals after mechanical stimulation. In mechanically stimulated cells, chloroplasts in the stimulus region (Fig. 8b) tended to move toward the base of the cell. Chloroplasts in regions both apical (Fig. 8c) and basal (Fig. 8a) to the stimulated region moved away from the stimulus for 2 h afterwards. In the later periods examined (4–6 h after stimulation), however, chloroplasts exhibited a tendency of recovery movement back to the stimulated area (Fig. 8).
Figure 6.
Tracking of chloroplast movement during the avoidance response. Start and stop positions of chloroplast tracks made over 1 h after a 1-min mechanical stimulation are linked by arrows (A). Movement of chloroplasts in the control cell without the stimulation are also presented (B). The diagram of needle tip indicates the site of the stimulation. The protonemal tip is on the right. Bar = 20 μm.
Figure 7.
Time course of chloroplast mobility after mechanical stimulation. A, Chloroplasts are divided into three groups with respect to their locations before mechanical stimulation: those located in the basal region (a), in the stimulus region (b), and in the apical region (c). Each region is 50 μm in length. B, Distances over which chloroplasts moved were measured in these three regions every hour during for 0 to 6 h after stimulation. The numbers of chloroplasts analyzed were: 14 (a), 23 (b), 17 (c), and 63 (control). Data were obtained from five protonemata. Control data from unstimulated cells are presented as a line with a se bar in each graph.
Figure 8.
Direction of chloroplast movement after mechanical stimulation. Direction of chloroplast movement in the basal region (a), in the stimulus region (b), and in the apical region (c) as shown in Figure 7A was analyzed hourly after mechanical stimulation. Chloroplasts that moved toward the apex (hatched bars) and toward the base (shaded bars) are shown as percentages. The percentage of chloroplasts that showed no movement is also shown on the right outside of the column. A control result is shown in d. Data were obtained from the same group of five protonemata as in Figure 7.
Nuclear Movement after Mechanical Stimulation
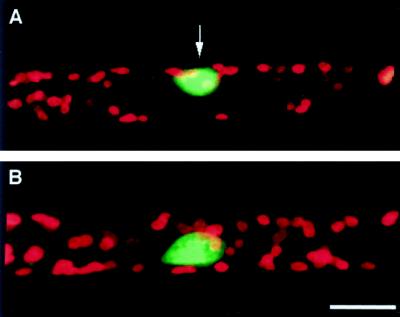
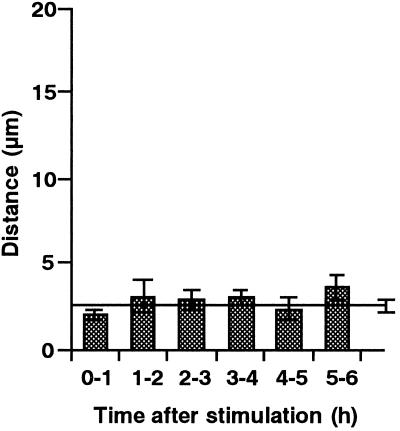
A nucleus can readily be identified in a single cell under the microscope. During the above experiments, in which nuclei were located at various distances from the stimulation point, no apparent movement of the nucleus in response to mechanical stimulation was observed. Therefore, the behavior of nuclei after mechanical stimulation was further investigated. Mechanical stimulation was applied for 1 min above the nuclear region of the cell, in the same way as for chloroplast avoidance analysis. No apparent avoidance response of nuclei was seen within 6 h after mechanical stimulation, while the chloroplasts around the nuclei moved away from the stimulation site. A nucleus could be clearly observed in the clear zone (Fig. 9). No significant change in the velocity or direction of nuclear movement was detected during these experiments (Fig. 10).
Figure 9.
Fluorescence micrographs of nuclei and chloroplasts after mechanical stimulation at the nuclear region. Two hours after mechanical stimulation in the nuclear region, a cell was stained with SYTO 11 (A). A cell without the stimulation was also stained and presented as a control (B). Micrographs are shown as combined images of fluorescence of SYTO 11 (green) and of chlorophyll (red). An arrow indicates the site of the stimulation. Bar = 20 μm.
Figure 10.
Time course of nuclear movement after mechanical stimulation. After stimulation in the nuclear regions of protonemata, distances over which nuclei moved were measured hourly for 0 to 6 h after stimulation. Each bar represents the mean ± se obtained from seven protonemata. Control data from unstimulated cells are presented as a line with a se bar (n = 7).
DISCUSSION
Touch-Induced Chloroplast Avoidance Response in A. capillus-veneris Protonemal Cells
In this study, cellular responses to mechanical stimulation in A. capillus-veneris protonemal cells were identified. We have clearly shown that the avoidance response of chloroplasts was induced by a stimulus applied from outside of the cell. It should be noted that no impalement with a capillary was necessary for the induction of the response. These results demonstrate, for the first time to our knowledge, that chloroplasts migrate away from a mechanically stimulated site. This chloroplast avoidance response was induced to a maximum degree even with a 0.3-min stimulus, the shortest period used in this study (Fig. 2), showing that the protonemal cells are very sensitive to mechanical stresses. It would be useful to know the threshold period for induction of the response. However, it was difficult to apply the stimulus for time periods shorter than 0.3 min using the present experimental system. It was also not possible to quantitate the strength of mechanical stimulation.
The Motile System Mediating Chloroplast Avoidance Movement
We found that cytochalasin B and BDM were effective and colchicine and APM were ineffective inhibitors of the mechanically induced avoidance response of chloroplasts. Although cytochalasin B and BDM also inhibited cytoplasmic streaming within 2 h at concentrations of 0.1 and 25 mm, respectively, the streaming recovered subsequently after replacement of the cells in inhibitor-free medium (data not shown). BDM is an agent that has been demonstrated to inhibit actomyosin ATPase and actin-myosin interaction in vitro (Horiuti et al., 1988; Higuchi and Takemori, 1989; Osterman et al., 1993; McKillop et al., 1994), but has not been shown to affect the organization of actin (Cramer and Mitchison, 1995; Grabski et al., 1998). Therefore, it seems that the avoidance movement of chloroplasts depends on the actomyosin motile system but not on the microtubule system. The photorelocation movement of chloroplasts is also due to the actin-based motile system in A. capillus-veneris (Kadota and Wada, 1992). The velocity of chloroplast movement during the avoidance response was close to that observed during photorelocation movement (Kagawa and Wada, 1996). Taken together, these data indicate that the same motile system may be used in both the photorelocation movement and the mechanorelocation movement of chloroplasts.
Chloroplast Avoidance Response Is a Directional Movement in A. capillus-veneris Protonemal Cells
Recently, chloroplasts in cells of the diatom Pleurosira laevis were found to translocate and gather around the nucleus following contact stimulation with a needle (Makita and Shihira-Ishikawa, 1997). In this cell, chloroplasts are located all around the cell periphery before the stimulation, but after the stimulation they move toward the nucleus, which resides in the center of the cell. The direction of chloroplast movement was always from the cell periphery toward the nuclear region, independent of the site of stimulation. In A. capillus-veneris protonemal cells, only the chloroplasts located near the stimulated site responded and moved away from the site. It is evident that the chloroplast response in a protonemal cell is a directional movement made to avoid the site of stimulation. Furthermore, the time required for translocation of chloroplasts was shown to be different between P. laevis and A. capillus-veneris. It took only 5 to 10 s in P. laevis to complete the response, while 0.5 to 2 h was necessary in A. capillus-veneris. The time required in A. capillus-veneris was close to that required for the photorelocation movement of chloroplasts in the same cell (Kadota and Wada, 1992). These results suggest that an unknown signal generated by mechanical stimulation arises and diffuses from the stimulated site and chloroplasts respond to this signal, resulting in the avoidance movement. Chloroplast avoidance seemed to be more rapid and to a greater extent when the cell was touched by the flank of a needle than by the tip (Fig. 1). This may suggest that the amount of signal is dependent on the amount of surface area contacted.
Behaviors of Other Organelles after Mechanical Stimulation
Movement of nuclei toward a wound site is known as “traumatotactic nuclear migration” (Nagai, 1993). Recently, Kennard and Cleary (1997) revealed that pressure focused on a small part of the cell causes “traumatotactic”-like nuclear migration to the site within 10 to 50 min after the onset of continuous stimulation. In A. capillus-veneris protonemal cells, migration of nuclei under conditions of chloroplast photomovement was reported (Kagawa and Wada, 1993). On the other hand, no apparent change in the location or mobility of a nucleus was observed within 6 h after mechanical stimulation. This result indicates that nuclei show neither “traumatotactic migration” toward the stimulated site nor avoidance movement from the site in A. capillus-veneris protonemal cells. Furthermore, no apparent change in cytoplasmic streaming could be observed before or after mechanical stimulation, and we could observe the movement of small vesicles through the clear zone (data not shown). Therefore, it seems that chloroplasts respond to mechanical stimulation independently from other organelles in A. capillus-veneris protonemal cells.
Possible Mechanisms and Purpose of the Response
The fact that only chloroplasts escape from the stimulated area implies that a specific motor system associated with chloroplasts is regulated by a gradient of some signal generated by mechanical stimulation. However, the molecular mechanism of mechanical perception and transduction of the avoidance response of chloroplasts are not understood at present. Ion channels activated by mechanical stimulation have been suggested to be a component of mechanotransduction steps (Morris, 1990). Mechano-induced transient increases in the cytosolic Ca2+ concentration have been detected at the tissue level in higher plants (Knight et al., 1991, 1992; Trewavas and Knight, 1994; Legue et al., 1997). Using the present experimental system for future studies, we might be able to monitor the cytosolic Ca2+ concentration before and after mechanical stimulation at the cellular level.
The reasons that chloroplasts move away from the mechanically stimulated site are mysterious, while in photorelocation movement it is understood that chloroplasts move toward an optimum light condition for photosynthesis. If the avoidance response has any ecological and/or physiological meaning, it should also be observable in other plant cells. Although it is not known whether chloroplasts in higher plant cells behave similarly, we have been able to observe the response in several fern species. The mechanically stimulated area would be hidden by an object and thus should suffer “shaded” conditions. If we assume that the response was acquired as a shade avoidance response independent of the photosensory mechanism, then its maintenance in these species makes sense.
ACKNOWLEDGMENTS
We thank Dr. Jane Silverthorn (University of California, Santa Cruz) for critical reading of the manuscript. We are also grateful to Dr. Haruko Kazama (International Christian University) for discussion and encouragement during the course of this study.
Footnotes
This work was supported by Grants-in-Aid for Scientific Research (to A.K. and M.W.) from the Ministry of Education, Science, Sports and Culture (Japan).
LITERATURE CITED
- Cramer LP, Mitchison TJ. Myosin I is involved in postmitotic cell spreading. J Cell Biol. 1995;131:179–189. doi: 10.1083/jcb.131.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C (1882) The Power of Movement in Plants. John Murray, London
- Grabski S, Arnoys E, Busch B, Schindler M. Regulation of actin tension in plant cells by kinases and phosphatases. Plant Physiol. 1998;116:279–290. [Google Scholar]
- Gross P, Julius C, Schmelzer E, Hahlbrock K. Translocation of cytoplasm and nucleus to fungal penetration sites is associated with depolymerization of microtubules and defence gene activation in infected, cultured parsley cells. EMBO J. 1993;12:1735–1744. doi: 10.1002/j.1460-2075.1993.tb05821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi H, Takemori S. Butanedione monoxime suppresses contraction and ATPase activity of rabbit skeletal muscle. J Biochem. 1989;105:638–643. doi: 10.1093/oxfordjournals.jbchem.a122717. [DOI] [PubMed] [Google Scholar]
- Horiuti K, Higuchi H, Umazume Y, Konishi M, Okazaki O, Kurihara S. Mechanism of action of 2,3-butanedione 2-monoxime on contraction of frog skeletal muscle fibers. J Muscle Res Cell Motil. 1988;9:156–164. doi: 10.1007/BF01773737. [DOI] [PubMed] [Google Scholar]
- Jaffe MJ. Planta. 1973;114:143–157. doi: 10.1007/BF00387472. [DOI] [PubMed] [Google Scholar]
- Kadota A, Wada M. Photoorientation of chloroplasts in protonemal cells of the fern Adiantum as analyzed by use of video-tracking system. Bot Mag Tokyo. 1992;105:265–279. [Google Scholar]
- Kadota A, Wada M. Cytoskeletal aspects of nuclear migration during tip-growth in the fern Adiantum protonemal cell. Protoplasma. 1995;188:170–179. [Google Scholar]
- Kagawa T, Wada M. Protoplasma. 1993;177:82–85. [Google Scholar]
- Kagawa T, Wada M. Brief irradiation with red or blue light induces orientational movement of chloroplasts in dark-adapted prothallial cells of the fern Adiantum. J Plant Res. 1994;107:389–398. [Google Scholar]
- Kagawa T, Wada M. Phytochrome- and blue-light-absorbing pigment-mediated directional movement of chloroplasts in dark-adapted prothallial cells of fern Adiantum as analyzed by microbeam irradiation. Planta. 1996;198:488–493. [Google Scholar]
- Kennard JL, Cleary AL. Pre-mitotic nuclear migration in subsidiary mother cells of Tradescantia occurs in G1 of the cell cycle and requires F-actin. Cell Motil Cytoskeleton. 1997;36:55–67. doi: 10.1002/(SICI)1097-0169(1997)36:1<55::AID-CM5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Knight MR, Campbell AK, Smith SM, Trewavas AJ. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors of cytoplasmic calcium. Nature. 1991;352:524–526. doi: 10.1038/352524a0. [DOI] [PubMed] [Google Scholar]
- Knight MR, Smith SM, Trewavas AJ. Wind-induced plant motion immediately increases cytosolic calcium. Proc Natl Acad Sci USA. 1992;89:4967–4971. doi: 10.1073/pnas.89.11.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legue V, Blancaflor E, Wymer C, Perbal G, Fantin D, Gilroy S. Cytoplasmic free Ca2+ in Arabidopsis roots changes in response to touch but not gravity. Plant Physiol. 1997;114:789–800. doi: 10.1104/pp.114.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita N, Shihira-Ishikawa I. Chloroplast assemblage by mechanical stimulation and its intercellular transmission in diatom cells. Protoplasma. 1997;197:86–95. [Google Scholar]
- McKillop DF, Fortune NS, Ranatunga KW, Greeves MA. The influence of 2,3-butanedione 2-monoxime (BDM) on the interaction between actin and myosin in solution and in skinned muscle fibers. J Muscle Res Cell Motil. 1994;15:309–318. doi: 10.1007/BF00123483. [DOI] [PubMed] [Google Scholar]
- Morris C. Mechanosensitive ion channels. J Membr Biol. 1990;113:93–107. doi: 10.1007/BF01872883. [DOI] [PubMed] [Google Scholar]
- Murata T, Wada M. Effects of colchicine and amiprophos-methyl on microfibril arrangement and cell shape in Adiantum protonemal cells. Protoplasma. 1989;151:81–87. [Google Scholar]
- Nagai R. Regulation of intracellular movements in plant cells by environmental stimuli. Int Rev Cytol. 1993;145:251–310. [Google Scholar]
- Osterman A, Arner A, Malmqvist U. Effects of 2,3-butanedione monoxime on activation of contraction and crossbridge kinetics in intact and chemically skinned smooth muscle fibers from guinea pig taenia coli. J Muscle Res Cell Motil. 1993;14:186–194. doi: 10.1007/BF00115453. [DOI] [PubMed] [Google Scholar]
- Trewavas A, Knight M. Mechanical signaling, calcium and plant form. Plant Mol Biol. 1994;26:1329–1341. doi: 10.1007/BF00016478. [DOI] [PubMed] [Google Scholar]
- Wada M, Furuya M. Photocontrol of the orientation of cell division in Adiantum. I. Effects of the dark and red periods in the apical cell of gametophytes. Dev Growth Differ. 1970;12:109–118. doi: 10.1111/j.1440-169x.1970.00109.x. [DOI] [PubMed] [Google Scholar]
- Wada M, Grolig F, Haupt W. Light-oriented chloroplast positioning: contribution to progress in photobiology. J Photochem Photobiol B Biol. 1993;17:3–25. [Google Scholar]
- Williamson RE. Organelle movement. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:181–202. [Google Scholar]
- Yatsuhashi H, Hashimoto T, Wada M. Dichroic orientation of photoreceptors for chloroplast movement in Adiantum protonemata. Non-helical orientation. Plant Sci. 1987a;51:165–170. [Google Scholar]
- Yatsuhashi H, Wada M. High-fluence rate responses in the light-oriented chloroplast movement in Adiantum protonemata. Plant Sci. 1990;68:87–94. [Google Scholar]
- Yatsuhashi H, Wada M, Hashimoto T. Dichroic orientation of phytochrome and blue-light photoreceptor in Adiantum protonemata as determined by chloroplast movement. Act Physiol Plant. 1987b;9:163–173. [Google Scholar]