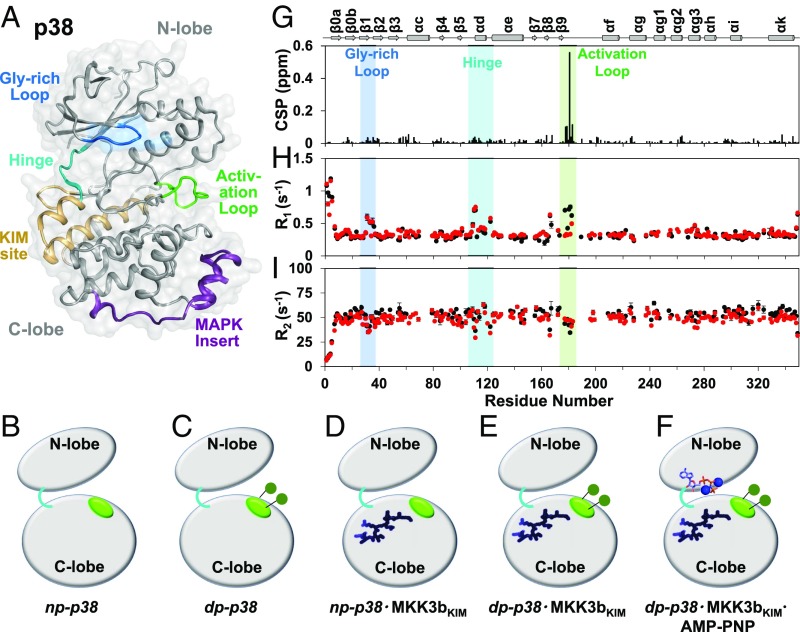
Fig. 1.
Phosphorylation affects fast timescale dynamics at the activation loop. (A) p38 adopts a typical kinase fold with an N- and a C-terminal lobe. Gly-rich loop, KIM-binding site, hinge, activation loop, and the MAPK-specific insert are highlighted. (B–F) Cartoon representation of the p38 states investigated in this work: (B) np-p38, (C) dp-p38, (D) np-p38⋅MKK3bKIM, (E) dp-p38⋅MKK3bKIM, and (F) np-p38⋅MKK3bKIM⋅AMP-PNP. Activation loop (green), KIM-peptide (dark blue), and AMP-Mg+2–binding sites (sticks) are highlighted. (G) Chemical-shift perturbations (CSP) upon activation-loop phosphorylation vs. residue number. (H) The 15N longitudinal relaxation rates (R1) and (I) transverse relaxation rates (R2) for np-p38 (black) and dp-p38 (red) vs. residue number. The Gly-rich loop (blue), hinge (cyan), and activation loop (green) are highlighted.