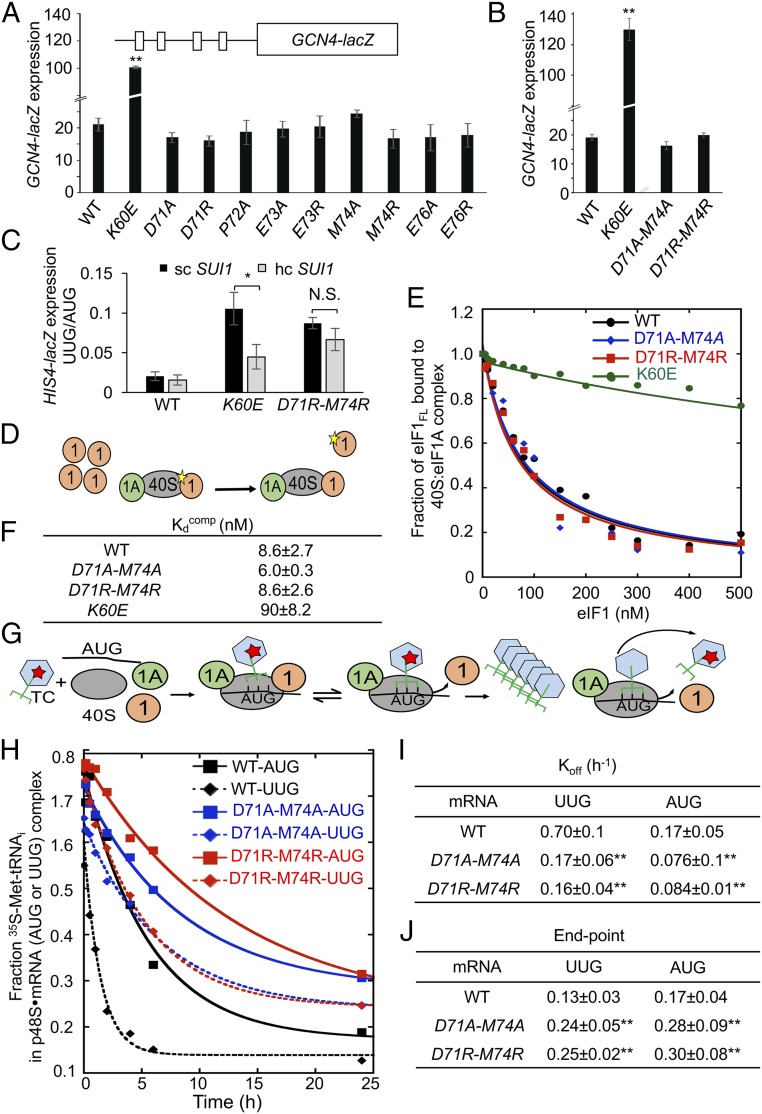
Fig. 4.
eIF1 Loop 2 substitutions do not impair eIF1–40S interaction or reduce TC recruitment in vivo but stabilize the closed/PIN conformation of the 48S PIC at UUG codons in vitro. (A and B) Transformants of JCY03 containing the indicated SUI1 alleles and a GCN4-lacZ reporter (p180) were assayed for β-galactosidase activities as in Fig. 2C. Mean expression levels and SEMs calculated from six to eight transformants of each strain are plotted. (C) Transformants of JCY03 containing WT SUI1 on an sc (pJCB01) or hc (pCFB04), K60E on an sc (pPMB02) or hc (pPMB39), and D71R-M74R on an sc (ATP98) or hc (ATP169) plasmid, and HIS4-lacZ reporters with AUG or UUG start codons, were assayed for β-galactosidase activities as in Fig. 2C. Asterisks in A–C indicate significant differences between mutant and WT as judged by a two-tailed, unpaired Student’s t test (*P < 0.05; **P < 0.01). (D–F) Measurement of eIF1 binding constants. Fluorescein-labeled WT eIF1 (5 nM) was prebound to 40S subunits (15 nM) in the presence of eIF1A (1 µM), mixed with increasing concentrations of unlabeled WT eIF1, eIF1-D71A-M74A, or eIF1-D71R-M74R, and the change in fluorescence anisotropy measured (D). One of two replicate experiments (E) from which mean Kdcomp values and average deviations were calculated (F). (G–J) Measurement of TC dissociation kinetics. Partial 48S complexes were assembled with radiolabeled WT TC, eIF1A, and model mRNA containing an AUG or UUG start codon, and with WT or mutant eIF1 proteins; they were chased with excess unlabeled TC for increasing periods of time; and the fraction of labeled Met-tRNAi bound to the PIC was determined (G). One of four replicate experiments (H) from which mean rate constants (I) and end points (J) (with SEMs) were calculated. Asterisks in I and J indicate significant differences between mutant and WT as judged by a two-tailed, unpaired Student’s t test (*P < 0.05; **P < 0.01).