Abstract
Rat pups readily form a 24-h associative odor preference after a single trial of odor paired with intermittent stroking. Recent evidence shows that this training trial, which normally increases AMPA receptor responses in the anterior piriform cortex both 3 and 24 h following training, induces a down-regulation of NMDA receptors 3 h later followed by NMDA receptor up-regulation at 24 h. When retrained with the same odor at 3 h, rat pups unlearn the original odor preference. Unlearning can be prevented by blocking NMDA receptors during retraining. Here, the mechanisms that initiate NMDA receptor down-regulation are assessed. Blocking mGluR receptors or calcineurin during training prevents down-regulation of NMDA receptors 3 h following training. Blocking NMDA receptors during training does not affect NMDA receptor down-regulation. Thus, down-regulation can be engaged separately from associative learning. When unlearning occurs, AMPA and NMDA receptor levels at 24 h are reset to control levels. Calcineurin blockade during retraining prevents unlearning consistent with the role of NMDA receptor down-regulation. The relationship of these events to the metaplasticity and plasticity mechanisms of long-term depression and depotentiation is discussed. We suggest a possible functional role of NMDA receptor down-regulation in offline stabilization of learned odor representations.
Keywords: metaplasticity, NMDA receptor, olfactory learning, piriform cortex, plasticity
Introduction
In rat pup odor preference learning, a single 10-min exposure to a novel odor paired with intermittent stroking produces a 24-h protein-synthesis-dependent odor preference (Grimes et al. 2011), which is associated with enhanced AMPA receptor (AMPAR) currents in olfactory inputs to both the olfactory bulb (OB) (Yuan and Harley 2012) and the anterior piriform cortex (aPC) (Fontaine et al. 2013; Morrison et al. 2013). AMPAR phosphorylation and increased AMPAR membrane insertion occur concomitantly with olfactory learning and memory in this model (Cui et al. 2011), consistent with a prediction of enhanced network representations with training. The neural changes and behavioral memory endure over multiple days with repeated spaced trials (Fontaine et al. 2013), whereas the odor representational network becomes more stable following spaced training in both the OB and aPC (Shakhawat et al. 2014).
In both OB and aPC, the NMDA GluN1 receptor levels are decreased 3 h following a single training trial (Lethbridge et al. 2012; Mukherjee et al. 2014) but then increase beyond control levels at 24 h in the aPC (Mukherjee et al. 2014). This contrasts with increases in AMPAR responses (likely due to increased membrane expression) at both time points (Morrison et al. 2013). In the aPC in vitro, long-term depression (LTD), normally difficult to induce in rat pup slices, is readily induced from slices taken at 3 h post odor preference training when NMDA receptors (NMDARs) are down-regulated (Mukherjee et al. 2014). Behaviorally, ‘unlearning’ occurs when a second odor preference training is given 3 h following the initial training (Mukherjee et al. 2014). Unlearning is specific to the trained odor since pairing a different odor with stroking at 3 h does not affect the learning of the new odor assessed 24 h later and does not disrupt memory for the originally trained preference (Mukherjee et al. 2014). The critical role of the reduced NMDAR expression in unlearning is demonstrated by blocking NMDARs prior to the second training event at 3 h. Whereas this prevents the original associative odor learning if given before the first training trial (Morrison et al. 2013), NMDAR blockade before the second trial at 3 h permits the original 24-h odor preference to be maintained rather than ‘unlearned’ (Mukherjee et al. 2014).
The present experiments test the potential mechanisms for NMDAR down-regulation recruited during the original training and implicated by earlier studies in metaplasticity, a process by which prior events temporally alter subsequent plasticity susceptibility (Abraham 2008; Hunt and Castillo 2012). The outcomes reveal that the activation of metabotropic glutamate receptors and calcineurin are required for NMDAR down-regulation. A model is presented supporting a functional role for NMDAR down-regulation in establishing stable memory networks.
Materials and Methods
Animals and Ethics Statement
Sprague Dawley rat pups of either sex (Charles River) were used in this study. Animals were bred and pups were born on-site at the animal care facility. Litters were culled to 12 pups on postnatal day 1 (PD1; day of birth is designated PD0). Dams were maintained with ad libitum access to food and water. All procedures were approved by the Institutional Animal Care Committee at Memorial University of Newfoundland adherent to the guidelines by the Canadian Council on Animal Care.
Behavioral Studies
Behavioral experiments were carried out in a temperature controlled room at ∼27°C and followed the previously established protocols (Fontaine et al. 2013; Mukherjee et al. 2014) as described below. One-way ANOVAs and post hoc Fisher tests or two-sample t-test were used to determine statistical significance throughout the experiments.
Odor Preference Training
PD6 rat pups were assigned to an odor plus stroking (O/S+) or an odor only (O/S−) condition. Pups were removed from the nest and placed on normal bedding for 10-min habituation. Pups receiving conditioning O/S+ were placed on peppermint-scented bedding (0.3 mL peppermint extract in 500 mL bedding) and vigorously stroked with a paintbrush for 30 s, followed by a 30-s rest, repeated for 10 min. Pups in the control condition O/S− were placed in peppermint-scented bedding for 10 min without being stroked. All pups were returned to the dam after training. A subset of O/S+ pups were re-trained at 3 h after the first training, following the same procedure as in the first training.
Three types of experiments were employed. First, O/S+ pups were trained with single naris occluded, followed by brain tissue collection at 3 h for NMDAR GluN1 measurement (Fig. 1). Another group was re-trained at 3 h and killed at 24 h for both GluN1 and GluA1 measurement (Fig. 7). Second, pups underwent aPC drug or vehicle infusions right before a single O/S+ training. These pups were either killed at 3 h for GluN1 measurement (Figs 2–4) or tested for odor preference the next day (Fig. 5). Third, pups underwent aPC drug or vehicle infusions before the re-training at 3 h following the first training. These pups were then tested for odor preference the next day (Fig. 6).
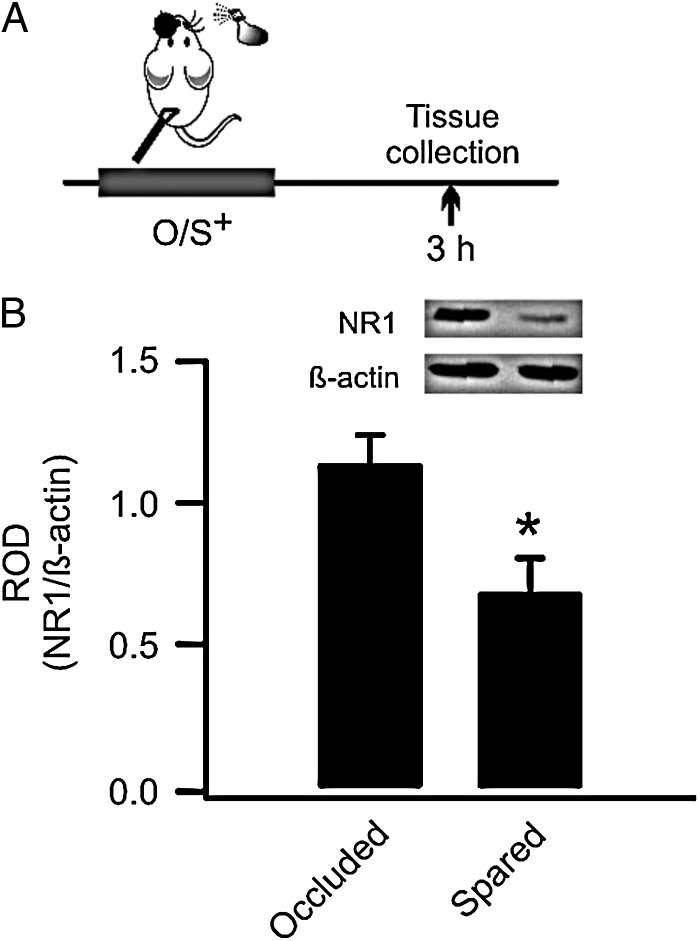
Figure 1.
Early odor preference learning in rat pups down-regulates synaptic GluN1 receptors in the anterior piriform cortex (aPC). (A) Schematics of the odor training and tissue-collection paradigm. O/S+: odor paired with stroking. (B) Relative optical density (ROD) of GluN1 expression (normalized to β-actin) in occluded and spared aPCs.
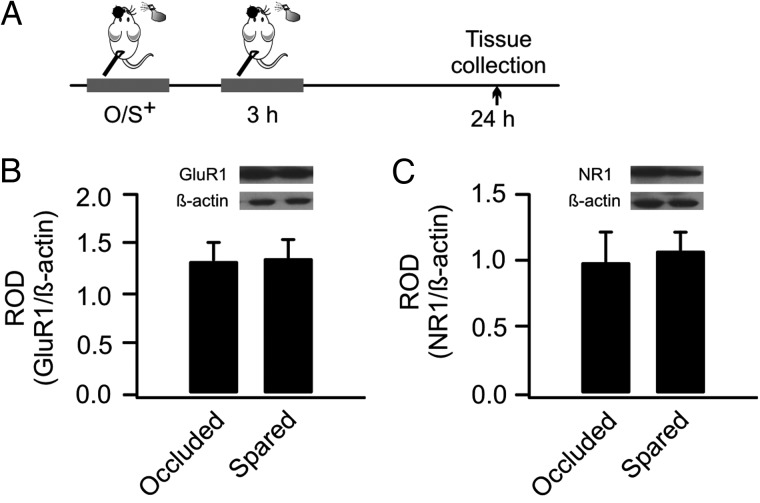
Figure 7.
Re-training resets AMPAR and NMDAR to the baseline level. (A) Schematics of the odor training and tissue collection paradigm. O/S+: odor paired with stroking. (B) ROD of GluA1 expression (normalized to β-actin) in occluded and spared aPCs. (C) ROD of GluN1 expression in occluded and spared aPCs.
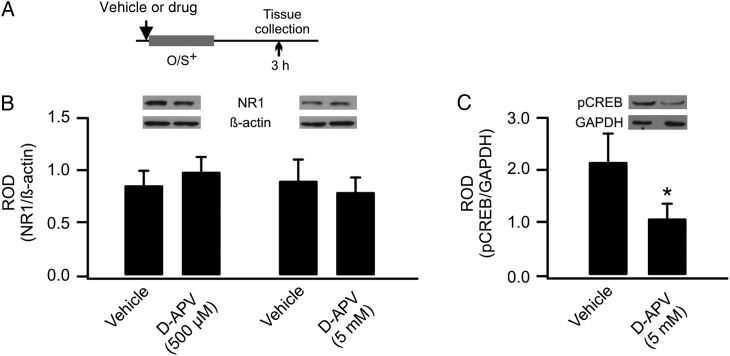
Figure 2.
GluN1 down-regulation is not dependent on NMDAR activation during early odor preference learning. (A) Schematics of the odor training and tissue collection paradigm. O/S+: odor paired with stroking. (B) ROD of GluN1 expression (normalized to β-actin) in vehicle- and D-APV-infused aPCs. (C) ROD of pCREB expression (normalized to GAPDH) in vehicle- and D-APV-infused aPCs.
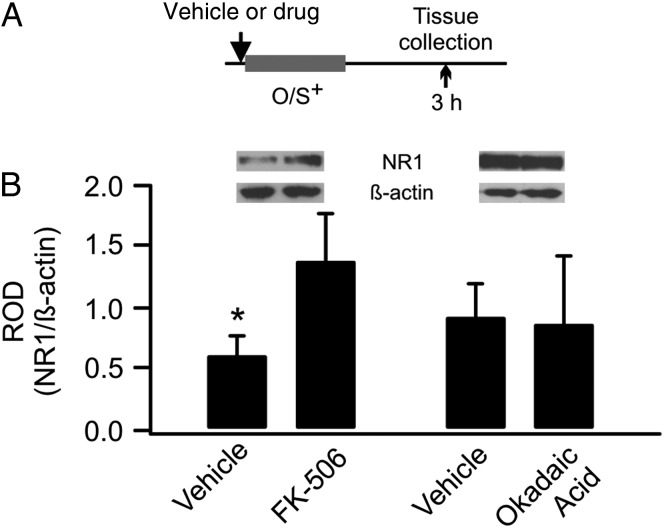
Figure 4.
GluN1 down-regulation is dependent on calcineurin signaling. (A) Schematics of the odor training and tissue collection paradigm. O/S+: odor paired with stroking. (B) ROD of GluN1 expression in vehicle-, FK-506-, or okadaic acid-infused aPCs.
Figure 5.
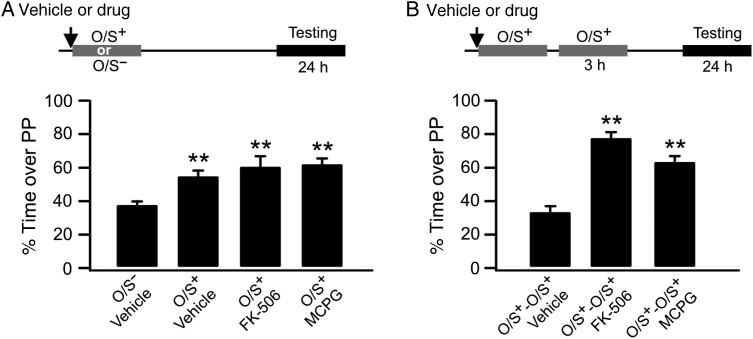
Inhibition of group I mGluR or calcineurin before first O/S+ training rescues early odor preference memory from re-training-induced unlearning. (A) Percentage of time spent over peppermint (PP)-scented bedding without re-training. O/S+: odor paired with stroking. O/S−: odor only without stroking. (B) Percentage of time spent over PP-scented bedding with re-training.
Figure 6.
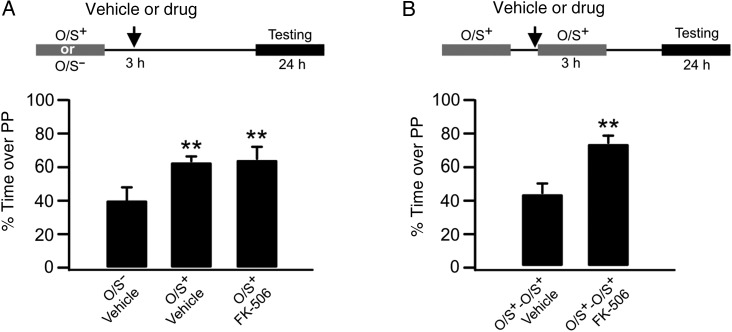
Inhibition of calcineurin before re-training rescues early odor preference memory. (A) Percentage of time spent over peppermint (PP)-scented bedding without re-training. O/S+: odor paired with stroking. O/S−: odor only without stroking. (B) Percentage of time spent over PP-scented bedding with re-training.
Odor Preference Testing
Twenty-four hours following the initial training, pups were tested for odor preference memory using a two-choice odor preference procedure. The testing apparatus was a stainless steel box (30 × 20 × 18 cm) placed over 2 training boxes. One box contained peppermint-scented bedding and the other contained normal, unscented bedding, separated by a 2-cm neutral zone. During testing, pups were removed from the dam and placed in the neutral zone. Times that pups spent over scented versus normal bedding were recorded in five 1-min trials, each separated by a 1-min rest in a clean cage. The percentage of the time spent over peppermint bedding over total time spent over either bedding was calculated for each pup.
Reversible Naris Occlusion
Nose plugs were constructed using polyethylene 20 (PE 20) tubing and silk surgical thread as described previously (Fontaine et al. 2013; Mukherjee et al. 2014). A small dab of 2% Xylocaine gel (AstraZeneca) was applied to the left naris of the pup, and the pup was let rest for ∼3–5 min before the plug was gently inserted in the left naris. After 10-min habituation, pups were assigned appropriate odor training. The nose plug was removed immediately following training and pups returned to dams.
Cannula Implantation and Intracerebral Infusion
Cannula implantation was carried out on PD5. Pups were anesthetized via hypothermia and placed in a stereotaxic apparatus in a skull flat position. A horizontal incision was made to expose the skull where 2 small holes were drilled. Two guide cannulas (Vita Needle, MA) with insect pins were inserted into the brain in specific coordinates for aPC (from Bregma: Anterior posterior: +2.5, Mediolateral: +3.5 and Depth: −5.5; [Morrison et al. 2013]), and cemented with dental acrylic to the skull. The skin was then sutured around the cannulas. The pups were recovered on warm bedding before being returned to the dams.
All drugs were infused into the aPC on PD6 either 20 min before the first training or before re-training. One microliter of a drug was injected bilaterally into the aPCs for behavioral experiments using a Hamilton syringe. In pups for quantitative immunoblotting, drugs were infused in 1 aPC and vehicles were infused in the contralateral hemisphere. The injection was over 4 min, and the syringe was left in place for another min before being gently withdrawn from the brain. The pups were returned to the dams for ∼5 min before habituation and training. Pharmacological agents used include an NMDAR antagonist D-AP5 (5 mM and 500 µM, dissolved in saline; Sigma–Aldrich), an mGluR1 antagonist AIDA (5 mm and 500 µM; dissolved in a small amount of 1 M NaOH and further diluted with saline, the same ratio of NaOH and saline was used as vehicle; Tocris), an mGluR5 antagonist MPEP (5 mm; 10% DMSO in saline; Tocris), an mGluR group I/II blocker MCPG (100 mm, dissolved in saline; Tocris), a calcineurin (phosphatase 2B) inhibitor FK-506 (5 mm, 10% DMSO in saline; Tocris) and a phosphatase 1/2A inhibitor okadaic acid (500 µm, 10% DMSO in saline; Calbiochem). All drug concentrations used are comparable with published results using in vivo brain infusions (Martin and Morris 1997; Ohno and Watanabe 1998; Christie-Fougere et al. 2009; Lethbridge et al. 2012; Ahmadi et al. 2013). The cannula locations were verified to be within the aPC during brain extractions. The spread of infusion was tested with 4% methylene blue dye in pilot experiments (<2 mm3; n = 6). We have also validated in previous studies (Morrison et al. 2013; Mukherjee et al. 2014) that the drug spread using the same infusion parameters and techniques was confined to the aPC.
Immunoblotting
Three hours following odor training, pups were decapitated, and aPCs were collected and flash-frozen on dry ice. For pCREB measurement, brains were taken 10 min following O/S+ training. Samples were stored at −80°C until further processing.
Synaptic Membrane Isolation
Purification of synaptic membrane followed previously published procedures (Goebel-Goody et al. 2009). Tissue samples were homogenized using a Teflon glass tissue homogenizer (Thomas Scientific) in ice-cold sucrose buffer (300 µL) containing (in mm): 320 sucrose, 10 Tris (pH 7.4), 1 EDTA, 1 EGTA, 1× complete protease inhibitor mixture and phosphatase inhibitor mixture (Roche). The homogenized samples were centrifuged at 1000 rpm for 10 min. The supernatant was spun at 10,000 rpm for 25 min to obtain a pellet, which was subsequently re-suspended in 120 µL sucrose buffer using a pestle mixing/grinding rod (Thomas Scientific) directly in the microfuge tube. Then, 8 volumes of a nonionic detergent Triton X-100 buffer (final 0.5% v/v) were added for detergent extraction. The Triton X-100 buffer contained (in mm) 10 Tris (pH 7.4), 1 EDTA, 1 EGTA, 1× protease and phosphatase inhibitors. This suspension was incubated at 4°C for 35 min with gentle rotation. Then, the suspension was centrifuged at 28 000 rpm for 30 min. The pellet (postsynaptic densities and synaptic junctions that are insoluble in Triton X-100, [Cotman and Taylor 1972]) was re-suspended in 100 µL of TE buffer containing 100 mm Tris (pH 7.4), 10 mm EDTA, 1% SDS, 1× protease and phosphatase inhibitors, sonicated, boiled for 5 min and stored at −80°C until use. Protein concentrations for each sample were determined by using a BCA protein assay kit (Pierce). The volume of lysate required to make 35 µg of protein for each sample was calculated.
Tissue Isolation for Phosphorylated CREB (pCREB) Measurement
APC tissue was placed in microcentrifuge tubes and homogenized with a manual motor pastel in 100 µL of lysis buffer containing 0.1% SDS, 1% NP-40, 20 mm PMSF, 10% glycine and 1.37 mm sodium chloride with 1 µL/mL leupeptin, 2 mm PMSF, 8.9 U/mL aprotinin and 1 mm sodium orthovanadate. The homogenate was centrifuged at 13 500 rpm for 15 min at 4°C. After determining the protein concentration, the clear lysate supernatant was stored at −80°C.
Western Blotting
A total of 100 µL lysate solution, sample buffer (0.3 M Tris–HCl, 10% SDS, 50% glycerol, 0.25% bromophenol blue, 0.5 M dithiothreitol) and dH2O were prepared and boiled for 2 min at 100°C. Samples were then loaded into lanes of a 7.5% SDS–PAGE gel, along with a protein ladder (Thermo Scientific). Sample separation occurred through SDS–PAGE, followed by transference to a nitrocellulose membrane (Millipore). Membranes were cut horizontally at the 72 kDa level, and the upper portion was probed with a rabbit antibody for GluN1 (1:2000, blocked in 5% Milk; Cell Signaling Technology) subunits, and the lower portion was probed for β-actin (1:5000, blocked in 5% skim milk; Cedarlane). A pCREB antibody (1:5000, Cell Signalling) and a control GAPDH antibody (1:7000, Cell Signalling) were used to measure pCREB levels. A GluA1 antibody (1:10 000, Cell Signalling) was used to probe AMPAR membrane levels. Membranes were incubated in primary antibody overnight at 4°C with continuous shaking. Next day membranes were washed 3 times for 5 min each with 1× TBST. Secondary antibodies bound to HRP were applied after the wash (1:10 000, anti-rabbit; Pierce) for 1 h, and membranes were then washed again with 1× TBST 3 times for 10 min each. Then, blots were washed in enhanced chemiluminescence Western blotting substrate (Pierce). Finally, blots were developed on X-ray film (AGFA). Films were scanned onto a computer using an image scanner (CanoScan LiDE 200), and the optical density (OD) of each band was measured using ImageJ software.
Each sample was normalized to the corresponding β-actin or GAPDH band that was run on the same gel. In pups that underwent lateralized odor training, each spared hemisphere was compared with its naris-occluded counterpart. In pups with drug infusions, the drug-infused hemisphere was compared with its vehicle-infused counterpart. Experimental values are reported as mean ± SEM of normalized optical densities. A paired t-test was used to evaluate differences in the mean optical densities between 2 groups.
Results
Synaptic GluN1 Down-Regulation Following Early Odor Preference Learning is mGluR-Dependent
Previously, using a synaptoneurosome preparation, we have shown that GluN1 subunits are down-regulated 3 h following O/S+ training in rat pups (Mukherjee et al. 2014). Synaptoneurosomes are composite structures enriched in synaptic proteins (Titulaer and Ghijsen 1997) and have been used to measure activity-dependent changes in AMPARs and NMDARs in the olfactory system (Quinlan et al. 2004; Cui et al. 2011; Lethbridge et al. 2012; Mukherjee et al. 2014). Here, we employed a protocol to further separate synaptic from extrasynaptic membrane compartments (Goebel-Goody et al. 2009) using a subcellular fraction approach followed by extraction with Triton X-100 as postsynaptic densities and synaptic junctions are shown to be insoluble in Triton X-100 (Cotman and Taylor 1972; Matus and Taff-Jones 1978). We validated this method by showing PSD-95 was particularly abundant in the synaptic fraction compared with either the extrasynaptic or cytosolic fractions (Supplementary Fig. 1). Our analysis revealed that 3 h following O/S+ training, the synaptic GluN1 subunit was significantly lower in the spared aPC (normalized OD: 0.69 ± 0.12) than in its occluded counterpart (1.14 ± 0.10, n = 5, t = 3.13, P = 0.04; Fig. 1). This confirms that GluN1 down-regulation occurs at synaptic membrane.
We next investigated whether synaptic GluN1 down-regulation is dependent on NMDARs. D-APV was infused in 1 aPC before the O/S+ training, whereas vehicle was infused into the other aPC. Two concentrations (500 µm and 5 mm) were tested. Neither the 500 µm (vehicle: 0.87 ± 0.14 vs. D-APV: 1.0 ± 0.13, n = 6, t = 0.97, P = 0.37) nor the 5 mm (vehicle: 0.92 ± 0.18 vs. D-APV: 0.81 ± 0.14, n = 6, t = 1.37, P = 0.23; Fig. 2A,B) dose altered GluN1 expression, suggesting GluN1 down-regulation is not NMDAR dependent. To validate the drug effect in aPC, we measured pCREB expressions following D-APV infusion and O/S+ training. The level of pCREB was lower in the D-APV-infused aPC (1.08 ± 0.27) than the control vehicle side (2.16 ± 0.53, n = 8, t = 2.61, P = 0.03, Fig. 2C), consistent with NMDAR-dependent CREB phosphorylation and odor preference learning (Lethbridge et al. 2012; Morrison et al. 2013). This result confirms the effectiveness of the drug infusion protocol.
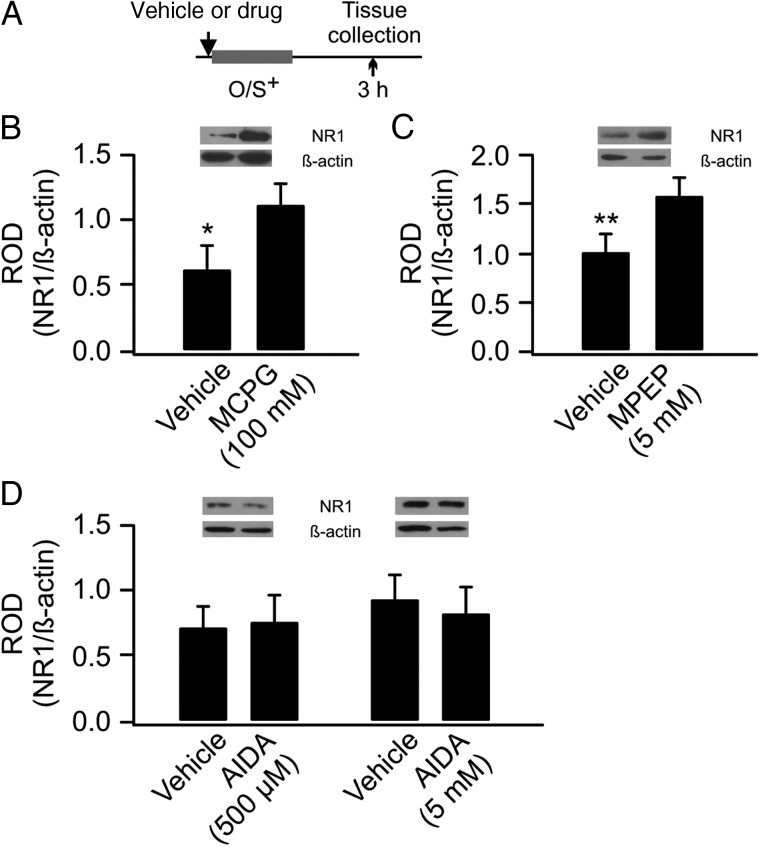
We then tested the potential involvement of mGluRs. When MCPG, an mGluR group I/II antagonist was infused, the GluN1 levels were significantly higher in the MCPG-infused aPC (1.12 ± 0.17) than the control side (0.63 ± 0.18, n = 8, t = 2.18, P = 0.03; Fig. 3A,B). This results suggest MCPG prevents GluN1 down-regulation following O/S+ training. To test the specific subtype of mGluRs involved, an mGluR5-specific antagonist MPEP (5 mm) was infused into the aPC. MPEP prevented down-regulation of GluN1 (MPEP: 1.59 ± 0.19 vs. vehicle: 1.00 ± 1.82, n = 8, t = 3.86, P = 0.006; Fig. 3C). However, an mGluR1-specific antagonist AIDA (500 µm and 5 mm) was ineffective. Neither the 500 µm (vehicle: 0.72 ± 0.16 vs. AIDA: 0.76 ± 0.20, n = 6, t = 0.48, P = 0.65) nor the 5 mm (vehicle: 0.93 ± 0.17 vs. AIDA: 0.82 ± 0.20, n = 6, t = 0.80, P = 0.46; Fig. 3D) dose altered GluN1 expression, suggesting GluN1 down-regulation is mediated by mGluR5, but not mGluR1.
Figure 3.
GluN1 down-regulation is dependent on mGluR activation. (A) Schematics of the odor training and tissue collection paradigm. O/S+: odor paired with stroking. (B) ROD of GluN1 expression (normalized to β-actin) in vehicle- and MCPG-infused aPCs. (C) ROD of GluN1 expression in vehicle- and MPEP-infused aPCs. (D). ROD of GluN1 expression in vehicle- and AIDA-infused aPCs.
Calcineurin Signaling is Involved in GluN1 Plasticity
Activation of mGluRs during early odor preference learning may trigger intracellular Ca2+ release, which could activate phosphatase pathways and lead to NMDAR dephosphorylation and internalization. We infused 2 phosphatase inhibitors – a calcineurin inhibitor, FK-506 (5 mm), and a phosphatase 1/2A inhibitor, okadaic acid (500 µm). FK-506 infusion prevented GluN1 down-regulation (vehicle: 0.62 ± 0.16 vs. FK-506: 1.39 ± 0.39, n = 8, t = 2.58, P = 0.04), whereas okadaic acid had no effect (vehicle: 0.93 ± 0.26 vs. FK-506: 0.88 ± 0.56, n = 6, t = 0.16, P = 0.88; Fig. 4).
As described GluN1 down-regulation following O/S+ training induces unlearning upon re-training at 3 h (Mukherjee et al. 2014). If GluN1 down-regulation is blocked, what would happen in the animals that are re-trained at 3 h following the initial O/S+ training? We infused either FK-506 or MCPG bilaterally into aPCs before the first O/S+ training and tested the effects on odor preference memory 24 h later, with or without 3-h re-training. When infused without re-training, neither drug affected odor preference learning compared with the O/S− control. A one-way ANOVA revealed significant differences among groups (F3,15 = 11.02, P < 0.001; Fig. 5A). The FK-506-infused group spent significantly more time on peppermint-scented bedding (60.80 ± 5.45%, n = 4) than the vehicle-infused O/S− control group (37.47 ± 2.88%, n = 6, t = 4.59, P < 0.001), and was not different from the vehicle-infused O/S+ learning group (55.58 ± 2.70%, n = 5, t = 0.99, P = 0.34), suggesting calcineurin inhibition does not interfere with early odor preference learning. Similarly, MCPG-infused pups also learned (62.12 ± 3.87, n = 4, t = 4.85, P < 0.001 compared with the vehicle O/S− group). However, when re-training occurred at 3 h after the first training, both drugs abolished unlearning. A one-way ANOVA revealed significant differences among groups (F2,10 = 54.57, P < 0.001; Fig. 5B). FK-506 infusion prevented unlearning (vehicle O/S+-O/S+: 33.06 ± 3.46%, n = 5, vs. FK-506 O/S+-O/S+: 78.49 ± 2.77%, n = 4, t = 10.13, P < 0.001). MCPG infusion also prevented unlearning in the re-trained group (MCPG O/S+-O/S+: 63.61 ± 3.11%, n = 4, t = 6.81, P < 0.001 compared with the vehicle O/S+-O/S+ group).
Calcineurin Signaling Mediates Unlearning
We have previously shown that unlearning with 3-h re-training is NMDAR-dependent (Mukherjee et al. 2014). To test whether a phosphatase-mediated depotentiation or LTD pathway mediates unlearning, we infused FK-506 into aPCs at 3 h with or without re-training. Without re-training, FK-506 infusion did not affect odor preference learning. A one-way ANOVA showed significant group effects (F2,11 = 4.52, P < 0.05; Fig. 6A). FK-506 infusion at 3 h without re-training (65.01 ± 7.39, n = 5) induced odor preference learning compared with the vehicle O/S− group (40.2 ± 7.64, n = 4, t = 2.70, P = 0.02). However, FK-506 infusion prevented unlearning when animals were re-trained at 3 h (vehicle O/S+-O/S+: 43.58 ± 6.38%, n = 6, vs. FK-506 O/S+-O/S+: 74.18 ± 3.71%, n = 4, t = 4.14, P < 0.01; Fig. 6B).
Unlearning Resets AMPARs and NMDARs to the Baseline Nonlearning Levels
AMPARs and NMDARs are both up-regulated at 24 h following a single O/S+ training (Morrison et al. 2013; Mukherjee et al. 2014). Here we measured how AMPARs and NMDARs changed at 24 h following the initial O/S+ training, with a 3-h re-training episode. Re-trained pups showed no difference of AMPAR GluA1 subunit expressions in the occluded (1.33 ± 0.18) and spared aPC (1.33 ± 0.17, n = 8, t = 0.32, P = 0.76; Fig. 7A and B). Similarly, NMDAR GluN1 subunits in the spared aPC (1.08 ± 0.13) were not different from the occluded hemisphere (1.00 ± 0.21, n = 8, t = 0.49, P = 0.64; Fig. 7A,C). These results suggest re-training at 3 h resets the levels of AMPAR and NMDAR to the baseline condition through metaplasticity.
Discussion
NMDAR Plasticity Following Early Odor Preference Learning
In the last decade, studies have begun to examine the activity-dependent regulation of NMDAR trafficking in in vitro preparations (Perez-Otano and Ehlers 2005; Lau and Zukin 2007). However, the molecular basis of activity-dependent NMDAR plasticity in intact, physiological conditions remains to be determined (Hunt and Castillo 2012). Our studies have provided among the first evidence that NMDAR activity is regulated by learning – early odor preference learning in rat pups induces an early transient and reversible down-regulation of NMDAR and a delayed up-regulation ((Mukherjee et al. 2014) and present results). Both rapid down-regulation and delayed up-regulation of NMDA receptors have been reported in in vitro models (Hunt and Castillo 2012).
AMPAR function is up-regulated in aPC at both the 3- and 24-h time points (Morrison et al. 2013); thus, there is a dissociation of GluN1 and GluA1 changes at the 3-h time point. While not typically reported, LTP of AMPAR-mediated and LTD of NMDAR-mediated changes have previously been described in nucleus accumbens neurons (Kombian and Malenka 1994).
Molecular Basis of Early GluN1 Down-Regulation with Single Trial Training
GluN1 down-regulation was not affected by NMDAR blockade through D-APV infusion. MCPG, a general group I and II mGluR antagonist, blocked learning-induced GluN1 down-regulation. Further testing with the mGluR5 antagonist, MPEP, also blocked GluN1 down-regulation. However, an mGluR1-specific inhibitor, AIDA, was not effective. This suggests that mGluR5 mediates the down-regulation of GluN1s. These receptors are strongly expressed in neocortical pyramidal neurons in rat pups of this age (Lopez-Bendito et al. 2002).
GluN1 down-regulation was also prevented by calcineurin inhibitor infusion, but not by the inhibition of phosphatase 1/2A. Activation of mGluRs during learning may activate calcineurin, which could dephosphorylate and enhance the activity of the tyrosine phosphatase STEP, which in turn dephosphorylates NMDAR GluN2B receptors. Dephosphorylation of GluN2B subunit promotes NMDAR internalization via the clathrin-mediated pathway as shown in other systems (Snyder et al. 2005). Our previous result demonstrating GluN1 down-regulation in a synaptoneurosome preparation (including both synaptic and extrasynaptic membranes) (Mukherjee et al. 2014) also favors an enhanced NMDAR internalization model, compared with increased lateral diffusion of the receptors (see Montgomery et al. [2005] for a discussion of the generality and power of NMDAR endocytosis as a plasticity mechanism).
Since early odor preference learning and AMPAR LTP at the synapses are critically dependent on NMDARs (Morrison et al. 2013), it was unexpected that an NMDAR antagonist that prevented pCREB expression after training was unable to prevent GluN1 down-regulation at 3 h. That is to say successful associative plasticity itself was not required to induce GluN1 down-regulation. This demonstrates that the mGluR5 pathway and the NMDAR pathways are engaged in parallel by associative training and can function independently when recruited by such training. The blockade of NMDARs prevents odor preference memory but does not prevent the ‘silent’ down-regulation of GluN1 receptors induced by training.
In contrast, blockade of calcineurin, which prevents GluN1 down-regulation driven by mGluR activation in this paradigm, typically enhances learning and memory (Christie-Fougere et al. 2009). In the odor preference model, the calcineurin inhibitor FK506 in the OB before training extends the duration of single trial memory and renders a suboptimal unconditioned stimulus optimal. In the present study, rat pups that had received FK506 before the initial training showed a strong odor preference memory following retraining at 3 h, rather than unlearning. It remains to be assessed whether the prevention of GluN1 down-regulation contributes to learning enhancement.
Calcineurin also appears to have a role in the NMDAR-mediated unlearning event itself. Blockade of calcineurin immediately before retraining at 3 h, despite normal GluN1 down-regulation at this time, renders the second training trial effective. This is likely due to calcineurin's role as a negative regulator of NMDAR plasticity, preventing the depotentiating or depressing NMDAR effect normally recruited by fewer receptors during the retraining.
Metaplasticity: LTD or Depotentiation?
Unlearning mediated by changes at the aPC synapse (decreased AMPAR LTP and inducible LTD) following early odor preference learning not only results from changes in NMDAR number but also depends on the NMDAR itself for its expression (Mukherjee et al. 2014). Blocking NMDARs during the retraining abolishes unlearning and permits the expression of odor preference memory (Mukherjee et al. 2014).
NMDARs have a role in both depotentiation (returning potentiated synapses to baseline) and LTD (reduced NMDAR currents promote LTD). Either of these mechanisms may underlie the unlearning effect observed here. Both are synapse-specific. Consistent with recruitment of LTD by retraining is the ability of a low-frequency protocol to recruit LTD in trained slices, but not untrained slices, when examined 3 h, but not 24 h, post-training (Mukherjee et al. 2014). On the other hand, resetting both NMDAR and AMPAR to control levels at 24 h by the ‘unlearning’ event, as seen here, is consistent with the reset function of depotentiation. Depotentiation is less likely to occur for strong LTP protocols (Woo and Nguyen 2003), and stronger odor preference training paradigms appear not to be associated with GluN1 down-regulation (unpublished observations). Single-trial odor preference learning is by definition a weak protocol since it only produces 24-h memory, but it is a long-term protein translation-dependent memory (Grimes et al. 2011), so these experiments are exploring the manipulation of mechanisms underlying the durations of long-term memories themselves.
Depotentiation is reported to depend on the GluN2A subunit whereas LTD is associated with the GluN2B subunit (Sanderson 2012). The known role of calcineurin in promoting endocytosis of the NMDAR through the GluN2B subunit (Snyder et al. 2001) suggests an LTD mechanism may be more likely here (but see Zhuo et al. [1999]).
These distinctions are of interest since metaplasticity is thought to recruit LTD, but not depotentiation (Abraham 2008). On the other hand since both metaplasticity and plasticity mechanisms are likely to be recruited with natural learning (Abraham 2008), it may not be possible to disentangle their contributions in odor preference learning. Most importantly, the synaptic changes induced by associative training lead to a temporally evolving and complex modulation of plasticity predispositions in the engaged circuitry. Selective manipulations of GluN2B and GluN2A subunits during the second training event might clarify the nature of the ‘unlearning’ mechanism.
Functional Roles of Synapse-Specific GluN1 Receptor Regulation with Associative Training
The up-regulation of GluN1 receptors at 24 h can readily be envisaged to provide a mechanistic basis for the memory strengthening effects of spaced learning (see Solomonia and McCabe [2015] for review of spaced learning effects). In the odor preference-learning model, longer-term memories and longer maintenance of AMPAR enhancement are associated with odor preference training spaced at 24 h (Fontaine et al. 2013). Imprinting in chicks also induces delayed GluN1 up-regulation in structures specifically associated with memory (Solomonia and McCabe 2015). Interestingly, the strength of the imprinting memory is positively correlated with the degree of delayed NMDAR up-regulation (McCabe and Horn 1988).
We suggest early down-regulation of the GluN1 receptor may be related to the removal of weak synapses in the learned odor representation during off-line reactivation. Such a function has been argued in several models (Hellier et al. 2007; Wiegert and Oertner 2013). Spontaneous network activity in conjunction with NMDAR-mediated LTD is thought to eliminate weaker synaptic connections (Wiegert and Oertner 2013). In one study (Le Be and Markram 2006), mGluR5s, but not NMDARs, were shown to be critically involved in the rewiring of neocortical microcircuitry through the selective elimination of weaker connections.
Recently, Arc+ cells have also been shown to express LTD mediated by mGluR group I (1/5) activation (Jakkamsetti et al. 2013). In the rat pup odor-learning model, we have shown that odors recruit Arc activation and that the odor representation by Arc+ cells following multiple spaced training is altered to have a larger proportion of stable or strong synaptic connections (Shakhawat et al. 2014). Thus, it is possible that one training effect of mGluR5 activation is to help shape important odor representations by reducing unstable connections during subsequent off-line activation as described for NMDA circuits in hippocampus (Hellier et al. 2007; Wiegert and Oertner 2013). GluN1 down-regulation would mediate unstable synapse elimination in odor representations following training.
Conclusions
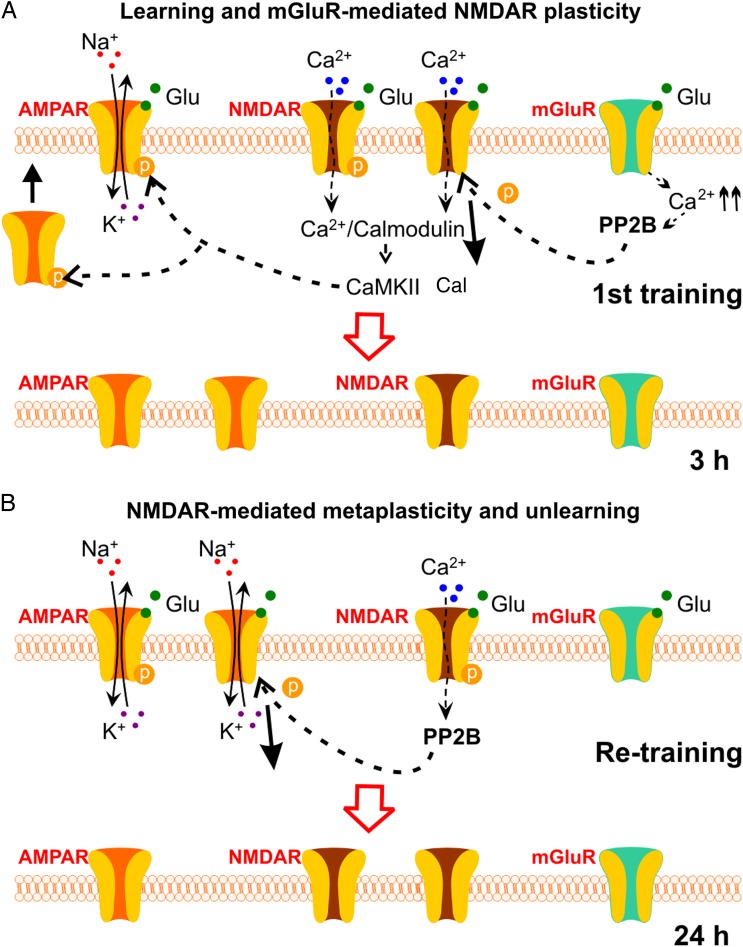
In this study, we characterize the molecular mechanisms of associative learning-induced NMDAR plasticity. GluN1 down-regulation was initiated by mGluR-mediated calcineurin signaling and inferred dephosphorylation and internalization of NMDARs. Blocking synapse-specific GluN1 down-regulation signaling prevents unlearning induced by a re-training episode. Unlearning during GluN1 down-regulation was shown to be mediated by an NMDAR-dependent calcineurin pathway inducing AMPAR internalization. Blocking GluN1 down-regulation signaling prevents unlearning induced by the re-training episode (see Fig. 8 for an illustration of the molecular mechanisms involved).
Figure 8.
Summary of pathways involved in NMDAR plasticity and metaplasticity in early odor preference learning in rats. (A) Learning and mGluR-mediated NMDAR plasticity. During the first training, large Ca2+ influx through NMDARs activates CaMKII, which phosphorylates AMPARs and facilitates AMPAR insertion into the synaptic membrane. Meanwhile, mGluR activation leads to Ca2+ release intracellularly and activates the PP2B pathway and dephosphorylates NMDARs. The latter results in NMDAR endocytosis and down-regulation at 3 h. Glu: glutamate; PP2B: phosphatase 2B (calcineurin). (B) NMDAR-mediated metaplasticity and unlearning. If re-training occurs at 3 h, moderate Ca2+ influx through fewer NMDARs results in PP2B-mediated AMPAR dephosphorylation and endocytosis. This re-sets the level of AMPARs to the original state before the first training.
We suggest learning-induced GluN1 down-regulation contributes to the increased stability of learned odor representations, whereas delayed GluN1 up-regulation supports the benefits of spaced training on memory duration.
Supplementary Material
Supplementary material can be found here.
Funding
This work is supported by a Canadian Institutes of Health Research operating grant (MOP-102624) to Q.Y.
Supplementary Material
Notes
Conflict of Interest: None declared.
References
- Abraham WC. 2008. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 9:387. [DOI] [PubMed] [Google Scholar]
- Ahmadi H, Nasehi M, Rostami P, Zarrindast MR. 2013. Involvement of the nucleus accumbens shell dopaminergic system in prelimbic NMDA-induced anxiolytic-like behaviors. Neuropharmacology. 71:112–123. [DOI] [PubMed] [Google Scholar]
- Christie-Fougere MM, Darby-King A, Harley CW, McLean JH. 2009. Calcineurin inhibition eliminates the normal inverted U curve, enhances acquisition and prolongs memory in a mammalian 3′-5′-cyclic AMP-dependent learning paradigm. Neuroscience. 158:1277–1283. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Taylor D. 1972. Isolation and structural studies on synaptic complexes from rat brain. J Cell Biol. 55:696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Darby-King A, Grimes MT, Howland JG, Wang YT, McLean JH, Harley CW. 2011. Odor preference learning and memory modify GluA1 phosphorylation and GluA1 distribution in the neonate rat olfactory bulb: testing the AMPA receptor hypothesis in an appetitive learning model. Learn Mem. 18:283–291. [DOI] [PubMed] [Google Scholar]
- Fontaine CJ, Harley CW, Yuan Q. 2013. Lateralized odor preference training in rat pups reveals an enhanced network response in anterior piriform cortex to olfactory input that parallels extended memory. J Neurosci. 33:15126–15131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. 2009. Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience. 158:1446–1459. [DOI] [PubMed] [Google Scholar]
- Grimes MT, Smith M, Li X, Darby-King A, Harley CW, McLean JH. 2011. Mammalian intermediate-term memory: new findings in neonate rat. Neurobiol Learn Mem. 95:385–391. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Grosshans DR, Coultrap SJ, Jones JP, Dobelis P, Browning MD, Staley KJ. 2007. NMDA receptor trafficking at recurrent synapses stabilizes the state of the CA3 network. J Neurophysiol. 98:2818–2826. [DOI] [PubMed] [Google Scholar]
- Hunt DL, Castillo PE. 2012. Synaptic plasticity of NMDA receptors: mechanisms and functional implications. Curr Opin Neurobiol. 22:496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakkamsetti V, Tsai NP, Gross C, Molinaro G, Collins KA, Nicoletti F, Wang KH, Osten P, Bassell GJ, Gibson JR et al. 2013. Experience-induced Arc/Arg3.1 primes CA1 pyramidal neurons for metabotropic glutamate receptor-dependent long-term synaptic depression. Neuron. 80:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombian SB, Malenka RC. 1994. Simultaneous LTP of non-NMDA- and LTD of NMDA-receptor-mediated responses in the nucleus accumbens. Nature 368:242–246. [DOI] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. 2007. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 8:413–426. [DOI] [PubMed] [Google Scholar]
- Le Be JV, Markram H. 2006. Spontaneous and evoked synaptic rewiring in the neonatal neocortex. Proc Natl Acad Sci USA. 103:13214–13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lethbridge R, Hou Q, Harley CW, Yuan Q. 2012. Olfactory bulb glomerular NMDA receptors mediate olfactory nerve potentiation and odor preference learning in the neonate rat. PLoS One. 7:e35024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bendito G, Shigemoto R, Fairen A, Lujan R. 2002. Differential distribution of group I metabotropic glutamate receptors during rat cortical development. Cereb Cortex. 12:625–638. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Morris RG. 1997. (R,S)-alpha-methyl-4-carboxyphenylglycine (MCPG) fails to block long-term potentiation under urethane anaesthesia in vivo. Neuropharmacology. 36:1339–1354. [DOI] [PubMed] [Google Scholar]
- Matus AI, Taff-Jones DH. 1978. Morphology and molecular composition of isolated postsynaptic junctional structures. Proc R Soc Lond B Biol Sci. 203:135–151. [DOI] [PubMed] [Google Scholar]
- McCabe BJ, Horn G. 1988. Learning and memory: regional changes in N-methyl-D-aspartate receptors in the chick brain after imprinting. Proc Natl Acad Sci USA. 85:2849–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery JM, Selcher JC, Hanson JE, Madison DV. 2005. Dynamin-dependent NMDAR endocytosis during LTD and its dependence on synaptic state. BMC Neurosci. 6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison GL, Fontaine CJ, Harley CW, Yuan Q. 2013. A role for the anterior piriform cortex in early odor preference learning: evidence for multiple olfactory learning structures in the rat pup. J Neurophysiol. 110:141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee B, Morrison GL, Fontaine CJ, Hou Q, Harley CW, Yuan Q. 2014. Unlearning: NMDA receptor-mediated metaplasticity in the anterior piriform cortex following early odor preference training in rats. J Neurosci. 34:5143–5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Watanabe S. 1998. Enhanced N-methyl-D-aspartate function reverses working memory failure induced by blockade of group I metabotropic glutamate receptors in the rat hippocampus. Neurosci Lett. 240:37–40. [DOI] [PubMed] [Google Scholar]
- Perez-Otano I, Ehlers MD. 2005. Homeostatic plasticity and NMDA receptor trafficking. Trends Neurosci. 28:229–238. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Lebel D, Brosh I, Barkai E. 2004. A molecular mechanism for stabilization of learning-induced synaptic modifications. Neuron. 41:185–192. [DOI] [PubMed] [Google Scholar]
- Sanderson TM. 2012. Molecular mechanisms involved in depotentiation and their relevance to schizophrenia. Chonnam Med J. 48:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhawat AM, Gheidi A, Hou Q, Dhillon SK, Marrone DF, Harley CW, Yuan Q. 2014. Visualizing the engram: learning stabilizes odor representations in the olfactory network. J Neurosci. 34:15394–15401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK et al. 2005. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 8:1051–1058. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. 2001. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 4:1079–1085. [DOI] [PubMed] [Google Scholar]
- Solomonia RO, McCabe BJ. 2015. Molecular mechanisms of memory in imprinting. Neurosci Biobehav Rev. 50:56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titulaer MN, Ghijsen WE. 1997. Synaptoneurosomes. A preparation for studying subhippocampal GABAA receptor activity. Methods Mol Biol. 72:49–59. [DOI] [PubMed] [Google Scholar]
- Wiegert JS, Oertner TG. 2013. Long-term depression triggers the selective elimination of weakly integrated synapses. Proc Natl Acad Sci USA. 110:E4510–E4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo NH, Nguyen PV. 2003. Protein synthesis is required for synaptic immunity to depotentiation. J Neurosci. 23:1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Harley CW. 2012. What a nostril knows: olfactory nerve-evoked AMPA responses increase while NMDA responses decrease at 24-h post-training for lateralized odor preference memory in neonate rat. Learn Mem. 19:50–53. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Zhang W, Son H, Mansuy I, Sobel RA, Seidman J, Kandel ER. 1999. A selective role of calcineurin aalpha in synaptic depotentiation in hippocampus. Proc Natl Acad Sci USA. 96:4650–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.