Figure 8.
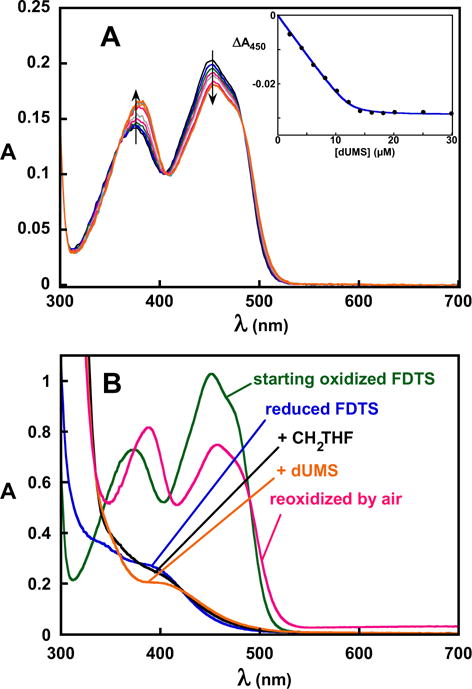
Binding and reactivity of dUMS in 0.1 M Tris-HCl, pH 8 at 25 °C. A, spectrophotometric titration of WT FDTS with dUMS. dUMS produced a flavin spectral change similar to that produced by dUMP. Fitting to Eq. 1 gave a Kd of 170 nM. B, dUMS failed to oxidize reduced FDTS in the presence of CH2THF. Anaerobic oxidized FDTS (green) was titrated stoichiometrically with dithionite to reduce the flavin (blue). Anaerobic addition of CH2THF perturbed the spectrum of the flavin (black), indicating that it formed a complex with the enzyme. Anaerobic addition of dUMS to the reduced FDTS-CH2THF complex also perturbed the spectrum (orange), indicating binding, but it failed to oxidize the enzyme. The flavin was then re-oxidized by exposure to air (red).
