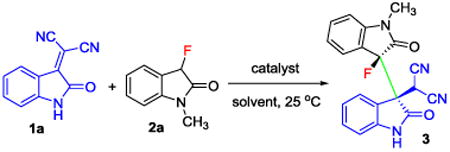
Table 1.
Optimization of the Michael addition of 3-fluorooxindole 2a to the isatylidene malononitrile 1a.a
| |||||
|---|---|---|---|---|---|
|
| |||||
| Entry | Catalyst | Solvent | Time (h) | Yield (%)b | drc |
| 1 | Et3N | THF | 2 | 99 | >99 : 1 |
| 2 | No base | H2O | 12 | nr | -- |
| 3 | Et3N | Acetone | 1 | 76 | >99 : 1 |
| 4 | Et3N | CH2Cl2 | 2 | 93 | >99 : 1 |
| 5 | Et3N | EtOH | 1 | 97 | >99 : 1 |
| 6 | Et3N | THF:H2O (1:1) | 2 | 99 | >99 : 1 |
| 7 | Et3N | EtOH:H2O (1:1) | 2 | 98 | >99 : 1 |
| 8 | Et3N | H2O | 3 | 99 | >99 : 1 |
| 9d | NaHCO3 | H2O | 18 | 16 | >99 : 1 |
| 10 | Na2CO3 | H2O | 18 | 45 | >99 : 1 |
| 11 | K2CO3 | H2O | 18 | 78 | >99 : 1 |
| 12e | Et3N | H2O | 2 | 99 | >99 : 1 |
| 13f | Et3N | H2O | 7 | 81 | >99 : 1 |
Reaction conditions: 0.2 mmol of 1a and 0.2 mmol of 2a in 0.5 mL of solvent, 10 mol% of catalyst, 25 °C,
Isolated yield,
Determined by 1H and 19F NMR,
0.5 mL of sat. NaHCO3,
20 mol% of Et3N,
5 mol% of Et3N.
