Table 1.
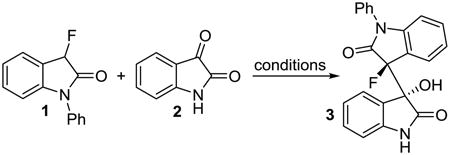
Optimization of the organocatalytic C-C bond formation with N-phenyl-3-fluorooxindole, 1, and isatin, 2.
| |||||
|---|---|---|---|---|---|
|
| |||||
| Entry | Solvent | Base (mol%) | Time (min) | Conversiona (%) | Diastereomeric ratioa |
| 1 | H2O | Et3N (20) | 120 | 93 | 24:1 |
| 2 | H2O | none | 120 | 0 | n/a |
| 3 | MeOH | Et3N (20) | 60 | 99 | 49:1 |
| 4 | MeOH/H2O (1:1) | Et3N (20) | 60 | 95 | 24:1 |
| 5 | EtOH | Et3N (20) | 60 | 96 | 24:1 |
| 6 | i-PrOH | Et3N (20) | 30 | 99 | 49:1 |
| 7 | i-PrOH | Et3N (10) | 30 | 99 | 49:1 |
General reaction conditions: Et3N (10-20 mol%) was added to a mixture of oxindole 1 (45.4 mg, 0.2 mmol) and isatin 2 (30.0 mg, 0.2 mmol) in 1.0 mL of the solvent at room temperature.
Based on 19F and 1H NMR analysis.
