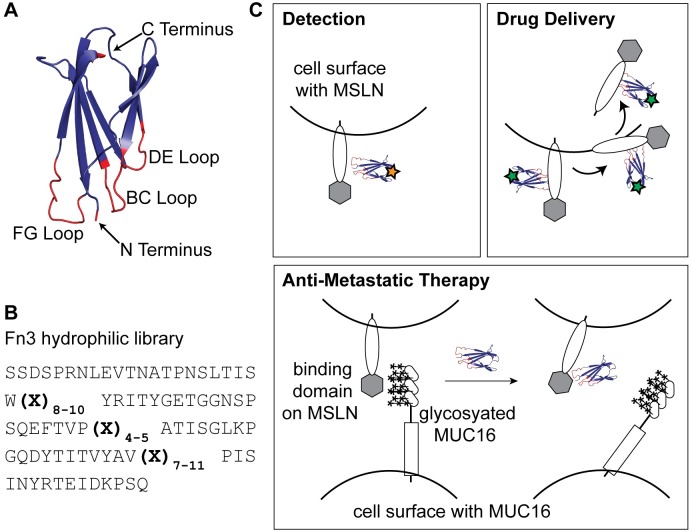
Fig 1. Approach to engineering Fn3 proteins to recognize tumor biomarker MSLN for diagnostic and therapeutic applications.
(A) The tenth domain of human fibronectin type III (Fn3) (PDB 1TTG) is a highly stable protein structure with three loops (BC, DE, and FG) broadly tolerant of mutation to confer novel binding properties. Structure was rendered in PyMOL. (B) We employed a previously developed hydrophilic Fn3 yeast surface display library [56] that incorporates a range of loop lengths and biased amino acid composition to mimic the diversity of naturally occurring antibody complementarity-determining regions. (C) Fn3 proteins that bind cell surface protein MSLN have numerous potential clinical applications, such as through diagnostic imaging, internalization for drug delivery, and metastatic reduction by blocking MSLN-MUC16 interactions. Stars represent conjugated imaging or therapeutic molecules.