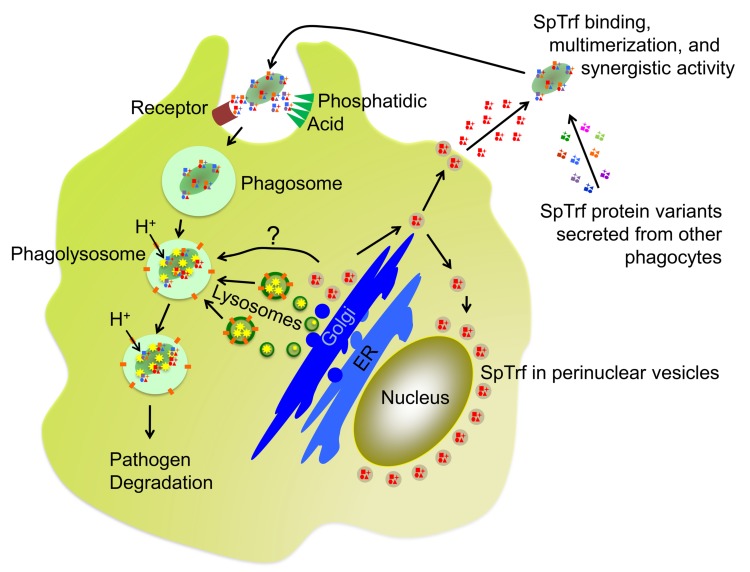
Fig 9. Putative trafficking and function of SpTrf proteins.
The current hypotheses of SpTrf protein function is that they are initially produced and stored in perinuclear vesicles of phagocytes. Note that a single SpTrf gene is expressed in a single phagocyte according to Majeske et al. [47], which would produce a few versions of the SpTrf protein (shown in red) resulting from RNA editing [54] and post translational processing [53]. SpTrf proteins are secreted from vesicles upon bacterial detection but vesicles are also hypothesized to fuse with phagolysosomes and SpTrf proteins may function in the degradation of phagocytosed microbes. After secretion, multiple SpTrf isoforms (indicated in multiple colors) mix in the CF and multimerize upon opsonization of bacteria that augments phagocytosis and may retard bacterial growth. How phagocytes recognize SpTrf proteins on the surface of microbes is not known, however, a speculative receptor (brown) is shown. SpTrf proteins may also bind to phosphatidic acid (PA; green triangles), which has been demonstrated for SpTrf-E1 [56]. The conical structure of PA and its clustering induced by SpTrf binding and multimerization is known to drive membrane curvature, which may augment phagocytosis. ER, endoplasmic reticulum.